Synonyms
Periodic chart
Introduction and Definition
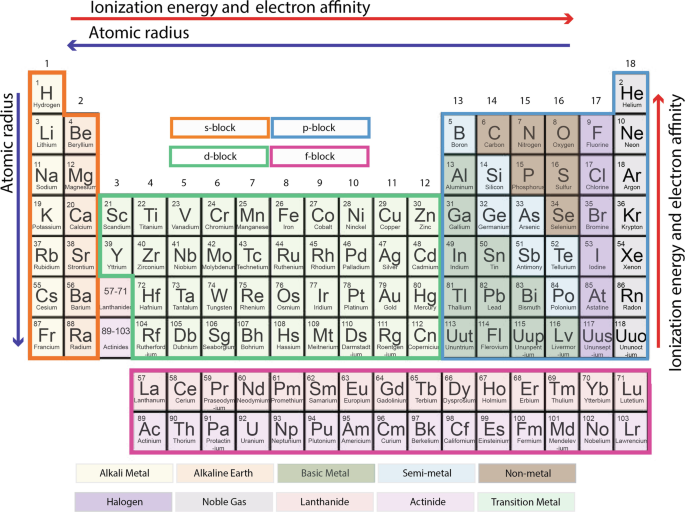
The modern periodic table is a schematic arrangement of all known elements, in order of increasing atomic number (i.e., number of protons in an element, its defining characteristic; Fig. 1) and hence their mass. The elegance of the periodic table is that the two-dimensional layout conveys far more than just atomic number and mass. Incorporated into the design is information about how electrons are arranged around the nucleus, the reactivities of elements, and patterns in the elements’ chemical properties. If one understands these aspects of the periodic table, it can also provide insight into how elements behave in Earth’s geochemical systems.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
De Chancourtois A-EB (1862) Mémoire sur un classement naturel des corps simples ou radicaux appelé vis tellurique. Comptes Rendus 54:757–761
Emsley J (2011) Nature’s building blocks: everything you need to know about the elements. Oxford University Press, Oxford
Goldschmidt VM (1925) Geochemische verteilungsgesetze der element, Part V. Isomorphie und polymorphie der sesquioxyde. Die lanthaniden kontraktion und ihre konsequenzen. Oslo
Goldschmidt VM (1926) Geochemische verteilungsgesetze der elemente. Skrifter Norske Videnskaps. Akad, Oslo
Goldschmidt VM (1937) The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the chemical society. J Chem Soc:655–673
Gray HB, Simon JD, Trogler WC (1995) Braving the elements. University Science, Sausalito
Green J (1959) Geochemical table of the elements for 1959. Geol Soc Am Bull 70:1127–1184
Harkins WD (1917) The evolution of the elements and the stability of complex atoms. J Am Chem Soc 39(5):856–879
Janet C (1929) The helicoidal classification of the elements. Chemical News 138:372–374
Kean S (2010) The disappearing spoon and other true tales of madness, love, and the history of the world from the periodic table of the elements. Back Bay Books, New York
Lodders K (2010) Solar system abundances of the elements. Astrophys J 591:1220–1247
McDonough WF, Sun S-S (1995) The composition of the Earth. Chem Geol 120:223–253
Mendeleev D (1869) Ueber die beziehungen der eigenschaften zu den atomgewichten der elemente [On the relationship of the properties of the elements to their atomic weights]. Zeitschrift für Chemie 12:405–406
Mendeleev D (1871) Die periodische gesetzmässigkeit der chemischen elemente. Zhurnal Russkoe Fiziko-Khimicheskoe Obshchestvo 3:25. Annalen der Chemie und Pharmacie Supplement 8:133–229. [German version, 1872]
Newlands JAR (1865) On the law of octaves. Chemical News 12:83
Oddo G (1914) Die molekularstruktur der radioaktiven atome. Z Anorg Chem 87:253–268
Railsback LB (2003) An earth scientist’s periodic table of the elements and their ions. Geology 31:737–740. http://geology.gsapubs.org/content/31/9/737.short; http://www.gly.uga.edu/railsback/PTPopups2.html
Scerri E (2006) The periodic table. Oxford University Press, Oxford
Seaborg G (1964) Plutonium: the ornery element. Chemistry 37(6):12–17
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this entry
Cite this entry
Harpp, K. (2018). Periodic Table. In: White, W.M. (eds) Encyclopedia of Geochemistry. Encyclopedia of Earth Sciences Series. Springer, Cham. https://doi.org/10.1007/978-3-319-39312-4_245
Download citation
DOI: https://doi.org/10.1007/978-3-319-39312-4_245
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39311-7
Online ISBN: 978-3-319-39312-4
eBook Packages: Earth and Environmental ScienceReference Module Physical and Materials ScienceReference Module Earth and Environmental Sciences