Abstract
Background
Cysteinyl leukotrienes (CysLT) are potent inflammation-promoting mediators, but remain scarcely explored in COVID-19. We evaluated urinary CysLT (U-CysLT) relationship with disease severity and their usefulness for prognostication in hospitalized COVID-19 patients. The impact on U-CysLT of veno-venous extracorporeal membrane oxygenation (VV-ECMO) and of comorbidities such as hypertension and obesity was also assessed.
Methods
Blood and spot urine were collected in “severe” (n = 26), “critically ill” (n = 17) and “critically ill on VV-ECMO” (n = 17) patients with COVID-19 at days 1–2 (admission), 3–4, 5–8 and weekly thereafter, and in controls (n = 23) at a single time point. U-CysLT were measured by ELISA. Routine markers, prognostic scores and outcomes were also evaluated.
Results
U-CysLT did not differ between groups at admission, but significantly increased along hospitalization only in critical groups, being markedly higher in VV-ECMO patients, especially in hypertensives. U-CysLT values during the first week were positively associated with ICU and total hospital length of stay in critical groups and showed acceptable area under curve (AUC) for prediction of 30-day mortality (AUC: 0.734, p = 0.001) among all patients.
Conclusions
U-CysLT increase during hospitalization in critical COVID-19 patients, especially in hypertensives on VV-ECMO. U-CysLT association with severe outcomes suggests their usefulness for prognostication and as therapeutic targets.
Similar content being viewed by others
Introduction
SARS-CoV-2 is a novel single-RNA encapsulated virus that is responsible for the recent pandemic disease known as COVID-19 [1, 2]. Patients with COVID-19 exhibit excessive inflammatory activity. This inflammatory response, frequently termed as “cytokine storm”, may worsen the progression of the infection through acute alveolar damage [3] and endothelial damage of the pulmonary vessels, with associated disseminated thrombosis, complement activation and microangiopathy [4, 5], leading to acute respiratory distress syndrome (ARDS), multiorgan damage and, eventually, death [6].
Although cytokines have been the most studied inflammatory mediators in COVID-19 pathophysiology, other mediators such as leukotrienes (LT) and cysteinyl leukotrienes (CysLT) have also potent inflammation-promoting actions that may be of special relevance in this clinical context [7]. CysLT are known for their role in inflammation in diseases such as asthma and allergic rhinitis [8, 9], being powerful bronchoconstrictors in humans. Indeed, they exert several actions relevant for respiratory pathology, being able to stimulate mucous secretion and bronchial hyperresponsiveness, increase microvasculature permeability and stimulate eosinophil infiltration [10,11,12]. In addition, our group recently showed that urinary CysLT were positively correlated with endothelial activation, inflammation, oxidative stress, and hepatic dysfunction markers in septic shock patients [13]. CysLT, namely leukotriene C4 (LTC4), leukotriene D4 (LTD4) and leukotriene E4 (LTE4), are arachidonic acid-derived eicosanoids synthesized by the 5-lipoxygenase (5-LOX)/LTC4 synthase pathway in immunocompetent cells (e.g., mast cells, neutrophils, eosinophils, basophils and monocytes) [14, 15]. In the circulation, LTC4 and LTD4 are rapidly metabolized to LTE4, which also has a short half-life [16, 17]. LTE4 is excreted in urine and changes in its urinary excretion are considered to be evidence of short-term changes in the formation rate of LTC4 [18]. The G protein-coupled CysLT1 and CysLT2 receptors, expressed in the outer cell membrane of immune and inflammatory cells, endothelial cells and platelets, mediate most CysLT effects relevant for immune responses, namely macrophage activation, inflammatory cytokine release and activation of the transcription factor nuclear factor kappa B (NF-κB) that regulates various genes related to inflammation, contributing overall to a hyperinflammatory and immune activation state [9, 19,20,21]. Due to the similarities between COVID-19-associated and non-associated respiratory pathology, there is a likely association between CysLT and the pulmonary pathology of COVID-19 [22]. However, they remain scarcely explored in this disease.
For all these reasons, we aimed to evaluate the relationship between CysLT profile and COVID-19 severity, focusing on their role as biomarkers for risk stratification and prognostication, as well as their plausibility for eventual therapeutic targeting. Importantly, we also assessed the impact of comorbidities associated with higher risk of critical disease and death, such as arterial hypertension and obesity [23,24,25,26,27], in severe and critical COVID-19 patients, as well as the effect of veno-venous extracorporeal membrane oxygenation (VV-ECMO) on CysLT, which has never been reported so far.
Materials and methods
Study design and population
The present study is part of a larger research project (RESEARCH 4 COVID-19 grant, project 519-reference number 613690173, “Unresolved inflammation and endotheliitis in severe COVID-19 patients: identification of risk stratification biomarkers and therapeutic targets”, FCT—Fundação para a Ciência e a Tecnologia) involving patients from the ward of the Service of Infectious Diseases and from the Intensive Care Unit (ICU) of the Service of Intensive Care Medicine and the Service of Infectious Diseases of a tertiary hospital (Centro Hospitalar Universitário de São João, CHUSJ). From September 2020 to February 2021, we consecutively recruited patients with a laboratory-confirmed diagnosis of SARS-CoV-2 infection, defined by a positive result on an RT-PCR assay of a specimen collected on a nasopharyngeal swab, who were hospitalized in the context of hypoxemic respiratory failure and symptomatic for > 1 day. Patients were excluded if they were under 18 years of age, were pregnant or lactating or had a history of vasculitis or connective tissue disease. Sixty-one patients (n = 61) were enrolled in this single-center cohort study, with the majority of them being recruited within 72 h of a positive RT-PCR result. Admission to the ward or ICU and the time for intubation and mechanical ventilation or VV-ECMO was based on clinical judgment according to leges artis. Patients were divided into two groups according to COVID-19 disease severity [28]: patients with severe COVID-19 admitted to the ward (n = 27) and patients with critical COVID-19 admitted to the ICU (n = 34). Severe COVID-19 was characterized by the presence of oxygen saturation < 90% on room air, signs of pneumonia or signs of severe respiratory distress, whereas critical disease was defined as patients presenting criteria for Acute Respiratory Distress Syndrome (ARDS), sepsis, septic shock, or other conditions that require life-sustaining therapies, according to the World Health Organization’s latest guidelines [28]. For analysis purposes, we only included the patients in whom we were able to collect both blood and spot urine samples at admission. In the severe COVID-19 group, we excluded one patient who lacked a urine sample at admission. Therefore, we had a final number of 26 severe COVID-19 patients and 34 patients with critical COVID-19. The group of patients with critical COVID-19 was further subdivided into two groups depending on whether or not they received VV-ECMO support: group of critically ill COVID-19 patients on VV-ECMO (critical COVID-19 on VV-ECMO, n = 17) and group of critically ill COVID-19 patients without VV-ECMO support (critical COVID-19, n = 17).
Due to the prospective nature of our sampling, we were able to capture a heterogeneous population of ward patients and ICU patients, some recruited before or during admission to ICU. Controls (n = 23) were recruited among healthy blood donor volunteers from the Service of Immunohemotherapy of CHUSJ before the COVID-19 pandemic. All eligible patients provided written informed consent to participate in the study. For ICU patients unable to give consent, this was solicited from their next of kin. These patients provided informed consent retrospectively, whenever possible. Blood donor volunteers provided oral informed consent. The study was conducted in accordance with the Guidelines for Good Clinical Practice and the 1975 Declaration of Helsinki after approval by the CHUSJ Health Ethics Committee [CES 75-16, with project amended specifically for inclusion of subjects with COVID-19, within the scope of a RESEARCH 4 COVID-19 grant from FCT (special support for rapid implementation projects for innovative response solutions to COVID-19 pandemic)].
Clinical data and sample collection
Patients were followed during their stay in the ward or ICU by the medical team of the project.
Throughout their hospitalization, regular physical examination of the patients was performed to assess major vital and clinical signs. For each patient, the medical team assessed the data regarding clinical and relevant demographic parameters, which were further anonymously coded to the project database, along with routine laboratory data, guaranteeing confidentiality. Illness severity was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems at ICU admission. ICU length of stay, total hospital length of stay and mortality at 30 days were also evaluated. The group of patients with critical COVID-19 on VV-ECMO included some patients who were previously hospitalized in the ICU of other hospitals before admission to the ICU of CHUSJ and that period was counted for the calculation of ICU length of stay. In addition, all patient groups included a few patients who were further transferred from CHUSJ to other hospitals and all consecutive period of hospitalization was counted for calculation of total hospital length of stay.
For all patients, blood and spot urine samples were collected at different time points throughout their hospital stay at CHUSJ, whenever possible: up to 48 h (days 1–2; admission), on days 3–4, on days 5–8 after admission and weekly thereafter until hospital discharge or after a negative result in RT-PCR COVID-19 test. All collections of critical COVID-19 patients on VV-ECMO were started after VV-ECMO initiation. Samples (blood and spot urine) from controls were collected at a single time point. All samples were processed within 1–2 h of collection and stored at − 80ºC until assayed.
Quantification of routine markers
Most routine laboratory analyses were performed at the Service of Clinical Pathology of CHUSJ. An Olympus Beckman Coulter® AU5400 automated clinical chemistry analyser (Olympus, Hamburg, Germany) was used for the quantification of serum CRP (S-CRP) by an immunoturbidimetric assay, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) by kinetic photometric assays, gamma glutamyltransferase (G-GT) by a kinetic colorimetric method, serum alkaline phosphatase (ALP), total and direct bilirubin and albumin by colorimetric assays, serum LDH (S-LDH) by a spectrophotometric assay and plasma and urinary creatinine by the colorimetric Jaffe method. Differential leukocyte count (leukocytes, neutrophils, eosinophils, monocytes and lymphocytes) was analyzed by flow-cytometry in an automated hematology analysis system (Sysmex 5000; Emilio de Azevedo Campos, Porto, Portugal), with subsequent calculation of neutrophil-to-lymphocyte ratio (NLR) and neutrophil-to-monocyte ratio (NMR).
Quantification of lactate, partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) was performed by arterial blood gas analysis. Fraction of inspired oxygen (FiO2) was obtained from oxygen administration device and oxygen dose information in the medical records and the PaO2/FiO2 ratio was calculated.
Quantification of urinary cysteinyl leukotrienes
CysLT were measured in unextracted spot urine samples (U-CysLT) by a competitive enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Cysteinyl Leukotriene ELISA kit, Cayman Chemical Company, Ann Arbor, MI, USA). The values of U-CysLT measured by this kit were previously shown to be well correlated with the values obtained by ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-MS/MS) [29]. U-CysLT concentrations were further corrected for urinary creatinine values.
Quantification of proinflammatory cytokines
Serum proinflammatory cytokines (serum tumor necrosis factor alpha, S-TNF-α; serum interleukin 1 beta, S-IL-1β; serum IL-6, S-IL-6) were evaluated by multiplex immunoassays using a Luminex 200TM xMAP™ analyser (Luminex Corporation, Austin, TX, USA), according to the protocol of Human High Sensitivity T Cell Magnetic Bead Panel (Milliplex® Map kit, Millipore Corporation, Billerica, MA, USA). Raw data analysis (mean fluorescence intensity) was performed using a standard five parameter logistic (5-PL) curve fit created by the Luminex xPONENT® Software (version 3.1).
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) or as median (25th percentile; 75th percentile) for data with normal or non-normal distribution, respectively, or as percentage. Results are graphically represented as Box-and-Whiskers plots. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [30] Statistical analysis was conducted using the GraphPad Prism 9 software (GraphPad Software, La Jolla, California, USA). Results were analyzed by unpaired Student’s t test or Mann–Whitney U test, for comparisons between two groups, or by one-way ANOVA followed by a Tukey’s multiple comparison test or a Kruskal–Wallis test followed by a Dunn’s post hoc test, for comparison between three groups, where appropriate. Categorical variables were analyzed by the Chi-Square test. Biomarkers evolution throughout the hospitalization was analyzed by Wilcoxon matched pairs signed rank test. Due to scarcity of samples at later time points, statistical analysis was only possible for results obtained until week 5 of hospitalization. Spearman’s correlation analysis was used to estimate correlations between sets of nonparametric data among all critically ill patients (critical COVID-19 and critical COVID-19 on VV-ECMO patients) throughout the first week of hospitalization (admission; days 3–4; days 5–8).
Repeated measures multivariate analyses were conducted to determine the relationship between U-CysLT (as dependent variable) and the COVID-19 patient group or patient comorbidities, adjusted for some confounders such as age and gender, among all COVID-patients during the first week of hospitalization, or between U-CysLT (as dependent variable) and hypertension, previous RAAS inhibitor treatment, eGFR, ICU length of stay, total hospital length of stay and 30-day mortality, adjusted for age and gender, among all critically ill patients during the first week of hospitalization.
The ability of U-CysLT to discriminate 30-day mortality in all COVID-19 patients or only in critical COVID-19 patient groups was evaluated by plotting receiver operating characteristic (ROC) curves and computing the area under the curve (AUC). All p values of < 0.050 were considered significant.
To prevent possible bias in clinical evaluation, all the patients were examined by the same medical team included in the project. To assure comparability of biomarkers assessment, samples from controls, severe COVID-19, critical COVID-19 and critical COVID-19 on VV-ECMO groups were evenly distributed in each assay plate. There were missing values in some biomarkers due to insufficient volume to perform sample processing, dilution tests and assays. We had no permission to measure routine clinical biomarkers in controls (blood donor volunteers), except for creatinine, or to have access to their hospital laboratory reports or clinical records. The final number per group for the biomarkers/parameters evaluated at admission was as following: APACHE II, n = 17 vs n = 17 (Critical vs Critical on VV-ECMO); SAPS II, n = 17 vs n = 17 (Critical vs Critical on VV-ECMO); lactate, n = 20 vs n = 15 vs n = 16 (Severe vs Critical vs Critical on VV-ECMO); PaO2/FiO2 Ratio, n = 26 vs n = 17 vs n = 17 (Severe vs Critical vs Critical on VV-ECMO); PaCO2, n = 24 vs n = 17 vs n = 17 (Severe vs Critical vs Critical on VV-ECMO); AST, n = 25 vs n = 16 vs n = 13 (Severe vs Critical vs Critical on VV-ECMO); ALT, n = 25 vs n = 15 vs n = 13 (Severe vs Critical vs Critical on VV-ECMO); ALP, n = 24 vs n = 16 vs n = 13 (Severe vs Critical vs Critical on VV-ECMO); G-GT, n = 24 vs n = 16 vs n = 14 (Severe vs Critical vs Critical on VV-ECMO); total bilirubin, n = 24 vs n = 16 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); direct bilirubin, n = 17 vs n = 10 vs n = 10 (Severe vs Critical vs Critical on VV-ECMO); LDH, n = 24 vs n = 8 vs n = 5 (Severe vs Critical vs Critical on VV-ECMO); albumin, n = 19 vs n = 14 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); S-CRP, n = 26 vs n = 17 vs n = 17 (Severe vs Critical vs Critical on VV-ECMO); S-TNF-α, n = 23 vs n = 26 vs n = 17 vs n = 17 (Controls vs Severe vs Critical vs Critical on VV-ECMO); S-IL-1β, n = 23 vs n = 26 vs n = 17 vs n = 17 (Controls vs Severe vs Critical vs Critical on VV-ECMO); S-IL-6, n = 22 vs n = 26 vs n = 17 vs n = 17 (Controls vs Severe vs Critical vs Critical on VV-ECMO); leukocytes, n = 26 vs n = 17 vs n = 16 (Severe vs Critical vs Critical on VV-ECMO); neutrophils, n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); eosinophils, n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); monocytes, n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); lymphocytes, n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); NLR (neutrophils/lymphocytes ratio), n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO); NMR (neutrophils/monocytes, n = 26 vs n = 17 vs n = 15 (Severe vs Critical vs Critical on VV-ECMO). In addition, the number of patients in each group decreased throughout hospitalization due to death, withdrawal of consent, hospital discharge or a negative RT-PCR COVID-19 test (Supplementary Figs. 1–3). Moreover, there were some patients in whom it was not possible to collect blood and/or urine samples at all time points throughout hospitalization due to medical/nurse team logistics or due to the lack of a sufficient urine excretion by the patients (in the case of urine collection) (Supplementary Figs. 1–3). To avoid biasing the results, no imputation for missing values was used.
Sample size was defined according to the primary objectives of our FCT funded RESEARCH 4 COVID-19 project that consisted in characterizing resolution of inflammation and endotheliitis. Based on preliminary evaluations of specialized proresolving mediators and endocan in healthy controls, patients with severe disease and critically ill patients, using power analysis, we calculated a sample size of 21 subjects per group to obtain an 80% power, at a 5% significance level (effect size-to-standard deviation ratio ca. 0.9). Since there was an elevated number of critically ill patients on VV-ECMO and a high heterogeneity of values between critically ill patients without VV-ECMO support vs those on VV-ECMO, we further divided the group of patients with critical COVID-19 into two groups: critically ill (without VV- ECMO) and critically ill on VV-ECMO. Despite this change, we had a total sample size of 83 subjects (i.e., about 4 times the 21 initially estimated), albeit with only 17 patients per group in the 2 critically ill groups. Reporting of the study conforms to STROBE statement along with references to STROBE and the broader EQUATOR guidelines [31].
Results
Population demographic, clinical and biochemical characterization
Demographic, clinical and biochemical characteristics of the subjects included in the study are presented in Table 1. In this study, 23 healthy controls and 60 patients, 26 with severe COVID-19, 17 with critical COVID-19 and 17 with critical COVID-19 on VV-ECMO were assessed. Patients with severe COVID-19 were older than controls (p < 0.010), while critically ill COVID-19 on VV-ECMO patients were significantly younger than severe and critically ill COVID-19 patients (p < 0.001 and p < 0.050, respectively). There was a male predominance in all groups, but there were no significant gender differences between groups. Arterial hypertension was the most prevalent comorbidity in severe and critically ill COVID-19 patients, while obesity was the most prevalent in critical COVID-19 on VV-ECMO patients, followed by arterial hypertension, although no significant differences were found between patient groups. We did not find differences in APACHE II and SAPS II scores between the groups of critical patients. Lactate concentration at admission also did not differ between the patient groups.
Regarding respiratory function, PaCO2 was significantly higher only in patients with COVID-19 on VV-ECMO (p < 0.001 vs severe COVID-19) and the PaO2/FiO2 ratio was significantly lower in both groups of critically ill patients compared to patients with severe COVID-19 (p < 0.001). Accordingly, and by definition, there was a higher need of mechanical ventilation, non-invasive ventilation and high-flow cannula oxygen in all critical COVID-19 patients (with or without VV-ECMO) when compared with severe COVID-19 patients (p < 0.001, p < 0.001 and p = 0.027 respectively).
There was a significant difference between groups regarding the number of patients who were previously treated with RAAS inhibitors (p = 0.017), with the critical COVID-19 on VV-ECMO group including a lower proportion (24%) of patients receiving a RAAS inhibitor prior to admission, compared to the severe and critical groups (58% and 71%, respectively). In both groups of critically ill patients, the RAAS inhibitor treatment was interrupted during hospitalization. Regarding the therapeutics initiated at admission, almost all patients were treated with dexamethasone and very few were treated with remdesivir with no differences between groups. There was a higher proportion of critically ill COVID-19 patients (with or without VV-ECMO) receiving antibiotics compared to severe patients, although this difference was not statistically significant (p = 0.063).
All COVID-19 patient groups exhibited significantly higher concentrations of inflammatory cytokines such as S-TNF-α, S-IL-1β, and S-IL-6 compared to controls. S-CRP concentration was higher in patients with critical COVID-19 on VV-ECMO compared to severe COVID-19 patients (p < 0.013). Patients with critical COVID-19 or with critical COVID-19 on VV-ECMO also showed significantly higher leukocyte count when compared to severe COVID-19 patients (p < 0.05 and p < 0.001, respectively), as well as higher neutrophil count (p < 0.010 and p < 0.001 vs severe COVID-19, respectively). Critical COVID-19 on VV-ECMO patients had higher eosinophil count when compared to critical COVID-19 group (p < 0.001). No differences were observed for monocyte and lymphocyte counts, but NLR was significantly higher in critical COVID-19 group and in patients with critical COVID-19 on VV-ECMO when compared to severe COVID-19 patients (p < 0.001 and p < 0.050, respectively) and NMR was significantly higher in patients with critical COVID-19 compared to severe COVID-19 patients (p < 0.05).
Patients with critical COVID-19 on VV-ECMO exhibited higher values of hepatic cholestatic parameters, namely G-GT (p < 0.050 vs severe COVID-19 and critical COVID-19) and direct bilirubin (p = 0.049). Critically ill patients on VV-ECMO also had significantly lower values of albumin when compared to both severe and critical COVID-19 groups (p < 0.001 and p < 0.050, respectively). No other significant differences were found between the three groups concerning any other hepatic parameters.
Both groups of critically ill patients had a longer length of stay in the ICU than severe patients (p < 0.001), taking into account that five severe COVID-19 patients needed a temporary upgrade of care to ICU in the first week of hospitalization [median ICU length of stay (25th percentile; 75th percentile): 11 (3; 36) days]. At ICU admission, those five patients had mean (± S.E.M.) APACHE II and SAPS II scores of 11 ± 2 and 28 ± 10, respectively. Moreover, the group of critical COVID-19 on VV-ECMO had a longer length of stay in ICU than the critical COVID-19 group, although this difference was not statistically significant. Both groups of critically ill patients had a longer total hospital length of stay when compared to severe COVID-19 patients (p < 0.001 and p < 0.010, respectively). No significant differences were observed in mortality within 30 days between COVID-19 patient groups in this population sample.
U-CysLT in all groups at admission
There were no significant differences in admission U-CysLT values between groups (p = 0.151) (Fig. 1).
Comparison of U-CysLT between COVID-19 patient groups during the first week of hospitalization
Patients with critical COVID-19 on VV-ECMO showed markedly higher U-CysLT values than severe COVID-19 patients at days 3–4 (p < 0.010) and at days 5–8 (p < 0.010) (Fig. 2).
Comparison of U-CysLT between COVID-19 patient groups during the first week of hospitalization. Results are presented in Box-and-Whiskers plot, severe: admission (n = 26), days 3–4 (n = 20), days 5–8 (n = 15); critical: admission (n = 17), days 3–4 (n = 16), days 5–8 (n = 13); critical on VV-ECMO: admission (n = 17), days 3–4 (n = 17), days 5–8 (n = 17). U-CysLT urinary cysteinyl leukotrienes, VV-ECMO veno-venous extracorporeal membrane oxygenation
U-CysLT profiles throughout hospitalization
In severe COVID-19 patients, U-CysLT values remained unchanged during hospitalization (Fig. 3A). On the other hand, both patients with critical COVID-19 and critical COVID-19 on VV-ECMO presented a rising pattern of U-CysLT. In the critical COVID-19 group, these values were significantly higher from days 3–4 until week 2 compared to admission (Fig. 3B), while in critical COVID-19 on VV-ECMO group, U-CysLT values were higher from days 3–4 until week 4 when compared to values on admission (Fig. 3C).
U-CysLT profiles throughout hospitalization in patients with severe COVID-19 (A), critical COVID-19 (B) and critical COVID-19 on VV-ECMO (C). Results are presented in Box-and-Whiskers plot, severe: admission (n = 26), days 3–4 (n = 20), days 5–8 (n = 15), week 2 (n = 7), week 3 (n = 1), week 4 (n = 1), week 5 (n = 1); critical: admission (n = 17), days 3–4 (n = 16), days 5–8 (n = 13), week 2 (n = 7), week 3 (n = 5), week 4 (n = 3), week 5 (n = 3); critical on VV-ECMO: admission (n = 17), days 3–4 (n = 17), days 5–8 (n = 17), week 2 (n = 13), week 3 (n = 10), week 4 (n = 9), week 5 (n = 4). U-CysLT urinary cysteinyl leukotrienes, VV-ECMO veno-venous extracorporeal membrane oxygenation
Impact of arterial hypertension and obesity on U-CysLT during the first week of hospitalization
In the critical COVID-19 on VV-ECMO group, U-CysLT values were higher in hypertensive patients compared to normotensive ones, but these differences only achieved statistical significance at days 5–8 (p = 0.027) (Table2). Regarding severe and critical COVID-19 patients, no significant differences on U-CysLT values were found between hypertensive and normotensive patients at any time point (Table 2).
Obesity was not associated with higher U-CysLT. In fact, in patients with critical COVID-19, we observed significantly lower admission U-CysLT values in obese subjects compared to non-obese ones (p = 0.021) (Table 2). No significant differences between obese and non-obese subjects were observed at other time points in severe, critical or critical COVID-19 on VV-ECMO patients (Table 2).
Correlation analyses in all critically ill patients during the first week of hospitalization
U-CysLT values were positively correlated with lactate at admission and were associated with a poorer respiratory function, evidenced by their positive correlation with PaCO2 at admission and inverse correlation with PaO2/FiO2 at days 3–4. U-CysLT were also associated with hepatic injury/dysfunction throughout the first week of hospitalization, presenting inverse correlations with albumin and positive correlations with AST and ALP. U-CysLT were also positively correlated with several inflammatory markers/parameters, namely S-CRP, S-TNF-α, S-IL-6, leukocyte count, neutrophil count, eosinophil count and NMR. Finally, we also observed positive correlations between U-CysLT during the first week of hospitalization and the hospital length of stay (both ICU and total length of stay) (Table 3).
U-CysLT during the first week of hospitalization in survivors and non-survivors at 30 days
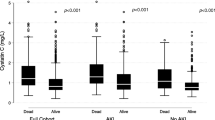
Non-survivor COVID-19 patients had significantly higher values of U-CysLT at admission (p < 0.010) and at days 3–4 (p < 0.010) compared to survivors (Fig. 4). On days 5–8, although non-survivors also presented higher values than survivors, this difference did not reach statistical significance (p = 0.148) (Fig. 4).
U-CysLT at admission, days 3–4 and 5–8 in COVID-19 survivors and non-survivors (mortality at 30 days). Results are presented in Box-and-Whiskers plot, survivors: admission (n = 52), days 3–4 (n = 47), days 5–8 (n = 40); non-survivors: admission (n = 8), days 3–4 (n = 6), days 5–8 (n = 5). U-CysLT urinary cysteinyl leukotrienes, VV-ECMO veno-venous extracorporeal membrane oxygenation
Repeated measures multivariate analysis
When considering all COVID-19 patients, we observed a significant positive association between U-CysLT and the most severe clinical presentation, i.e., the critical COVID-19 on VV-ECMO group (P = 0.001) (Table 4). We did not find any significant association of U-CysLT with comorbidities (data not shown).
Among all critical COVID-19 patient groups, higher U-CysLT values were significantly associated with higher ICU and total hospital length of stay (p < 0.001 and p = 0.013, respectively) (Table 4). Furthermore, survival at 30 days was associated with significantly lower values of U-CysLT (p = 0.035) (Table 4).
Performance of U-CysLT as a predictor of mortality at 30 days
We found that U-CysLT had a good ability of discriminating mortality at 30 days among all COVID-patients (AUC (U-CysLT): 0.734 [95% CI: 0.644–0.824], p = 0.001) (Fig. 5A) or in critical COVID-19 patient groups (AUC (U-CysLT): 0.692 [95% CI: 0.572–0.811], p = 0.022) (Fig. 5B).
Discussion
Our major findings were that U-CysLT significantly increased throughout hospitalization in both groups of critical COVID-19 patients, with the highest values being observed in those on VV-ECMO support. Furthermore, in VV-ECMO patients, arterial hypertension was also associated with significantly higher U-CysLT values at the end of the first week of hospitalization. Importantly, correlation and repeated measures multivariate analysis showed that, among critical patients, U-CysLT values during the first week of hospitalization were positively associated with prognostic parameters such as ICU length of stay, total hospital length of stay and mortality. This relationship with mortality at 30 days was also evidenced by the markedly higher U-CysLT values during the first hospitalization week in non-survivors when compared to survivors and corroborated by ROC curve analysis that confirmed a good ability of U-CysLT to predict 30-day mortality in COVID-19 patients.
As other causes of ARDS, COVID-19 pneumonia requiring VV-ECMO has a high mortality rate [32,33,34,35] but these patients are still scarcely studied. In our study, VV-ECMO patients showed the highest values of U-CysLT on the first week of hospitalization. Although aging has been linked to an imbalance in the production of arachidonic acid metabolites, with a shift favoring CysLT proinflammatory actions [36], it cannot explain our results since patients on VV-ECMO were the youngest COVID-19 patients, which is in accordance to guidelines and similar to that observed in other series of COVID-19 patients on VV-ECMO support [37, 38]. In fact, the younger age of our VV-ECMO patients might have been a major determinant for the low mortality observed in this group. In contrast, the significantly older age of patients with severe COVID-19 might explain the similar mortality found between severe and critical COVID-19 patients, since age appears to be the most relevant risk factor for critical disease and death, with a significantly increased risk at each life decade [39]. In VV-ECMO patients, severe hypoxia and obesity predominance could be partially responsible for the activation of the LT pathway, as already described for in vitro immune and endothelial cells exposed to intermittent hypoxia [40, 41] and in the severest stages of obstructive sleep apnea (OSA) [42, 43]. Body mass index (BMI) appears to be an independent predictor of urinary LTE4 values in obese (BMI ≥ 30 kg/m2) or overweight (25 < BMI < 30 kg/m2) OSA patients, even after adjustment for OSA severity [44]. Furthermore, adipocytes exhibit the ability to synthetize CysLT [45, 46]. However, when comparing U-CysLT in obese and non-obese patients within COVID-19 patient groups, obesity was not associated with higher values of these mediators and in critical COVID-19 patients, it was even associated with lower U-CysLT.
Arterial hypertension has been established as a major risk factor for disease severity and mortality associated with COVID-19, while being also a predictor of poor outcomes in COVID-19 patients [23,24,25], probably by dysregulation of the renin–angiotensin–aldosterone system (RAAS) and the association with inflammation and immune responses [47]. In our cohort, similarly to other series of COVID-19 critical patients [48], we found that hypertension was the most prevalent comorbidity among severe and critical COVID-19 groups and the second most frequent comorbidity in critical COVID-19 on VV-ECMO group. Noteworthy, our results showed that among critical on VV-ECMO patients, those who were hypertensive had higher U-CysLT values at the end of the first week of hospitalization. LTs are known to play a variety of roles in the cardiovascular system. Specifically, CysLT cause constriction of the smooth muscle, increase capillary permeability and can even decrease cardiac inotropism and coronary blood flow [49, 50]. Recent studies have shown that 5-LOX-derived proinflammatory mediators, including CysLT, may be involved in vascular remodeling contributing to elevated systemic vascular resistance and consequently to higher blood pressure in mice models [50]. Evidence has also shown that selective CysLT1 receptor antagonists were able to mitigate angiotensin II-mediated vasoconstriction in several animal and human models [51,52,53], therefore, suggesting a strong involvement of CysLT in the pathogenesis of angiotensin II-induced hypertension [54]. Given the relationship between CysLT and angiotensin II, along with the fact that treatment with RAAS inhibitors was interrupted during hospitalization in ICU patients, we could hypothesize that the maintenance, whenever possible, of antihypertensive treatment with RAAS inhibitors might be potentially beneficial for critical COVID-19 patients on VV-ECMO.
Both critical COVID-19 groups, but not severe patients, showed a rising pattern of U-CysLT values throughout hospitalization. This might have been caused by an upregulation of 5-LOX in critical COVID-19, but not in severe patients, as previously suggested by other authors who observed an increase in 5-LOX-derived mediators in the sera of ICU COVID-19 patients at day 4 post-admission [55]. Raised concentrations of 5-LOX-derived leukotrienes, such as LTB4 and LTE4, have also been evidenced in the bronchoalveolar lavage fluid of intubated COVID-19 patients [56].
In our study, critical COVID-19 groups also exhibited significant positive correlations of U-CysLT values with several systemic inflammatory markers and parameters, namely with the proinflammatory cytokines IL-6 and TNF-α, with CRP and with total leukocytes, neutrophils, eosinophils and NMR ratio. Interestingly, this relationship of U-CysLT with the inflammatory status was far more pronounced in critical COVID-19 patients than in septic shock patients recently evaluated by our group [13]. This suggests that U-CysLT could be more implicated in COVID pathogenesis, as also denoted by their association with prognosis. Interestingly, critical patients on VV-ECMO showed a higher number of eosinophils which are a known major source of CysLT [57]. CysLT mediate various eosinophil functions, namely their differentiation, maturation and survival, as well as chemotaxis and protein secretion, with most of the abovementioned functions being mediated by CysLT1 receptor [58]. Accordingly, CysLT1 receptor antagonists are able to reduce eosinophil recruitment seen in allergic inflammation of the airways [8, 59]. Both eosinopenia and eosinophilia seem to be associated with the dysregulated immune response in COVID-19 patients [60]. While eosinopenia has been associated with poor outcomes in initial acute phases of COVID-19 [60], eosinophil-mediated lung inflammation seems to contribute to the development of critical forms of the disease [61]. Overall, our findings seem to establish a connection between the hyperinflammatory state in critical COVID-19 patients and CysLT, especially during disease progression.
Dysregulation and overactivation of the immune system are probable mechanisms for liver injury in COVID-19 patients [62]. High concentrations of AST, ALT, LDH, total bilirubin and decreased albumin concentrations have been observed in severe forms of COVID-19 disease and are associated with hepatic dysfunction [63]. A cholestatic pattern, such as G-GT and ALP elevation, has also been described in COVID-19 patients [64]. Indeed, we noticed this same pattern in our critical COVID-19 on VV-ECMO patients, with raised concentrations of G-GT and direct bilirubin, as well as lower concentration of serum albumin. Noteworthy, in critical COVID-19 patient groups, U-CysLT were also associated with hepatic injury/dysfunction, being positively correlated with AST and ALP and inversely correlated with albumin. Importantly, we also recently evidenced a positive association between U-CysLT and hepatic injury in septic shock patients, thus reinforcing the contribution of these mediators to liver damage [13].
Ninety-four percent of patients in critical groups were under dexamethasone treatment according to hospital protocol and treatment guidelines [28]. Therefore, we could not evaluate the impact of dexamethasone on U-CysLT values in COVID-19 patients. Nonetheless, several studies have described an inability of both topical and systemic corticosteroids to modulate overall secretion of CysLTs by immune cells [65,66,67,68]. In addition, critical groups had a higher percent of patients under antibiotic treatment. Co-infection conditioning antibiotic treatment could be a confounder for the hyperinflammatory state and increased CysLT values. In fact, both lipoteichoic acids from Gram-positive and endotoxin from Gram-negative bacteria appear to induce CysLT production [69, 70]. On the other hand, antibiotics are known modulators of the inflammatory response and their immunomodulatory properties have been described in inflammatory respiratory diseases [71]. Further studies should therefore assess the impact of co-infection and antibiotic treatment on CysLT profile in COVID-19 patients.
U-CysLT values did not distinguish clinical severity at admission, appearing to have a more delayed kinetics in COVID-19 pathophysiology as evidenced by their rising pattern along hospitalization in critical patient groups. Importantly, U-CysLT values during the first week of hospitalization were consistently positively associated with relevant prognostic markers such as ICU length of stay, total hospital length of stay and mortality at 30 days. Non-survivors presented significantly higher values of U-CysLT during the first week of hospitalization and ROC curve analysis showed good performance of U-CysLT to predict short-term mortality among all patients and in critical patient groups. Thus, measurements of U-CysLT throughout the first week of hospitalization might be useful for risk stratification and prognostication, although this should be further studied in larger patient populations.
Overall, our findings are in accordance with the strong association between the hyperimmune state and COVID-19 progression and mortality [6], reinforcing CysLT as plausible mediators in this process, and, most relevantly, as potential therapeutic targets, as previously proposed [7, 72].To date, only a few studies examined CysLT involvement in COVID-19 pathophysiology. One study detected high concentrations of LTE4 in bronchoalveolar lavage fluid of patients with critical COVID-19 requiring mechanical ventilation [56]. Another study showed exacerbated macrophage production of CysLT three to five months after onset of non-severe COVID-19 [73]. Moreover, the role of proinflammatory eicosanoids in critical COVID-19 was recently highlighted [74]. Importantly, montelukast, an antagonist of CysLT1 receptor, was shown to inhibit platelet activation induced by plasma of COVID-19 patients [75]. This study also suggested that montelukast, if administered in the early phase of disease, could mitigate lung damage in COVID-19 patients by targeting inflammation and immune response, in addition to limiting the thrombotic phenomena that are observed in this disease [75]. In fact, two retrospective studies have already demonstrated that asthmatic patients under treatment with montelukast had fewer episodes of COVID-19 [76] or less episodes of clinical deterioration [77]. Just recently, a retrospective controlled cohort study also showed that the combined treatment with dexamethasone and CysLT receptor antagonists provided a survival advantage in COVID-19 patients with low oxygen saturations [78]. Clinical trials are already on place to test montelukast’s effectiveness on COVID-19 treatment, including COSMO [79] and E-SPREZANZA trials [80] and also a Portuguese randomized, controlled, parallel, open-label trial [81].
Major strengths of our study comprise the measurement of U-CysLT throughout hospitalization and the analysis of an extensive panel of biomarkers related to inflammatory and multisystemic manifestations of COVID-19, as well as the assessment of U-CysLT relationship with severity and prognostic parameters in hospitalized patients, especially in ICU and VV-ECMO patients where investigation is very limited. The use of non-invasive samples and a simple methodology to quantify U-CysLT, along with its consistent association with prognostic parameters, reinforce the clinical usefulness of this biomarker. Limitations of the study include its single-center design, which may condition the existence of a selection bias derived from consecutive recruitment, as well as the small number of patients included. In addition, we were not always able to collect blood and urine samples at all time points from all patients due to the high burden of clinical work during COVID-19 pandemic and also due to patient withdrawal of consent associated with the fear and psychological distress in hospitalized COVID-19 patients.
In conclusion, U-CysLT increases during hospitalization in patients with critical COVID-19, particularly in those on VV-ECMO support. Arterial hypertension, but not obesity, seems to contribute to the raised U-CysLT values at the end of the first week of hospitalization in critically ill patients on VV-ECMO. Importantly, among all critically ill patients, U-CysLT values along the first week of hospitalization were positively associated with outcomes such as ICU length of stay and total hospital length of stay. Moreover, a significant relationship between U-CysLT and mortality at 30 days was also confirmed, with U-CysLT values at first week of hospitalization showing good performance to predict short-term mortality among all patients and in critically ill patients. Thus, they may represent important biomarkers for risk stratification and prognostication in COVID-19, as well as therapeutic targets, especially in critically ill patients.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Triggle CR, Bansal D, Ding H, Islam MM, Farag EABA, Hadi HA, et al. A Comprehensive Review of Viral Characteristics, Transmission, Pathophysiology, Immune Response, and Management of SARS-CoV-2 and COVID-19 as a Basis for Controlling the Pandemic. Front Immunol. 2021;12:631139.
Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215: 108427.
Li S, Jiang L, Li X, Lin F, Wang Y, Li B, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.138070.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–8.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74.
Funk CD, Ardakani A. A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Front Pharmacol. 2020;11:1214.
Peters-Golden M. Expanding roles for leukotrienes in airway inflammation. Curr Allergy Asthma Rep. 2008;8:367–73.
Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochim Biophys Acta. 2011;1810:1096–102.
Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–55.
Henderson WR Jr. The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–97.
Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet. 1993;341:989–90.
Reina-Couto M, Santos-Oliveira M, Pereira-Terra P, Silva-Pereira C, Quelhas-Santos J, Duarte A, et al. Urinary cysteinyl leukotrienes as biomarkers of endothelial activation, inflammation and oxidative stress and their relationship with organ dysfunction in human septic shock. Biomedicines. 2022;10:2845.
Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36(6):689-703.
Theron AJ, Steel HC, Tintinger GR, Gravett CM, Anderson R, Feldman C. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function. J Immunol Res. 2014;2014:608930.
Hammarström S, Bernström K, Orning L, Dahlén SE, Hedqvist P. Rapid in vivo metabolism of leukotriene C3 in the monkey Macaca irus. Biochem Biophys Res Commun. 1981;101:1109–15.
Keppler D, Huber M, Baumert T, Guhlmann A. Metabolic inactivation of leukotrienes. Adv Enzyme Regul. 1989;28:307–19.
Maclouf J, Antoine C, De Caterina R, Sicari R, Murphy RC, Patrignani P, et al. Entry rate and metabolism of leukotriene C4 into vascular compartment in healthy subjects. Am J Physiol. 1992;263:H244–9.
Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med. 2007;357:1841–54.
Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–10.
Tahan F, Jazrawi E, Moodley T, Rovati GE, Adcock IM. Montelukast inhibits tumour necrosis factor-alpha-mediated interleukin-8 expression through inhibition of nuclear factor-kappaB p65-associated histone acetyltransferase activity. Clin Exp Allergy. 2008;38:805–11.
Aigner L, Pietrantonio F, Bessa de Sousa DM, Michael J, Schuster D, Reitsamer HA, et al. The leukotriene receptor antagonist montelukast as a potential COVID-19 therapeutic. Front Mol Biosci. 2020;7:610132.
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2001227.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–42.
Leiva Sisnieguez CE, Espeche WG, Salazar MR. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55:2001148.
Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev. 2021;37: e3377.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21: e13128.
Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370: m3379.
Bood JR, Sundblad BM, Delin I, Sjodin M, Larsson K, Anderson SD, et al. Urinary excretion of lipid mediators in response to repeated eucapnic voluntary hyperpnea in asthmatic subjects. J Appl Physiol. 1985;2015(119):272–9.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53.
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
Giraud R, Legouis D, Assouline B, De Charriere A, Decosterd D, Brunner ME, et al. Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol Rep. 2021;9: e14715.
Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. Extracorporeal Membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. Asaio j. 2021;67:485–95.
Gangemi S, Pescara L, D’Urbano E, Basile G, Nicita-Mauro V, Davì G, et al. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol. 2005;40:612–4.
Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. Asaio J. 2020;66:707–21.
Bertini P, Guarracino F, Falcone M, Nardelli P, Landoni G, Nocci M, et al. ECMO in COVID-19 patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2022;36:2700–6.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184:124–31.
Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a–5p. J Immunol. 2010;184:3878–88.
Stanke-Labesque F, Pépin JL, de Jouvencel T, Arnaud C, Baguet JP, Petri MH, et al. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J Lipid Res. 2012;53:1944–51.
Stanke-Labesque F, Pépin JL, Gautier-Veyret E, Lévy P, Bäck M. Leukotrienes as a molecular link between obstructive sleep apnoea and atherosclerosis. Cardiovasc Res. 2014;101:187–93.
Stanke-Labesque F, Bäck M, Lefebvre B, Tamisier R, Baguet JP, Arnol N, et al. Increased urinary leukotriene E4 excretion in obstructive sleep apnea: effects of obesity and hypoxia. J Allergy Clin Immunol. 2009;124(364–70):370.e1-2.
Bäck M, Sultan A, Ovchinnikova O, Hansson GK. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res. 2007;100:946–9.
Mothe-Satney I, Filloux C, Amghar H, Pons C, Bourlier V, Galitzky J, et al. Adipocytes secrete leukotrienes: contribution to obesity-associated inflammation and insulin resistance in mice. Diabetes. 2012;61:2311–9.
Peng M, He J, Xue Y, Yang X, Liu S, Gong Z. Role of hypertension on the severity of COVID-19: a review. J Cardiovasc Pharmacol. 2021;78:e648–55.
Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–83.
Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–72.
Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010;86:243–53.
Stanke-Labesque F, Devillier P, Veitl S, Caron F, Cracowski JL, Bessard G. Cysteinyl leukotrienes are involved in angiotensin II-induced contraction of aorta from spontaneously hypertensive rats. Cardiovasc Res. 2001;49:152–60.
Shastri S, McNeill JR, Wilson TW, Poduri R, Kaul C, Gopalakrishnan V. Cysteinyl leukotrienes mediate enhanced vasoconstriction to angiotensin II but not endothelin-1 in SHR. Am J Physiol Heart Circ Physiol. 2001;281:H342–9.
Stanke-Labesque F, Devillier P, Bedouch P, Cracowski JL, Chavanon O, Bessard G. Angiotensin II-induced contractions in human internal mammary artery: effects of cyclooxygenase and lipoxygenase inhibition. Cardiovasc Res. 2000;47:376–83.
Stanke-Labesque F, Hardy G, Vergnaud S, Devillier P, Peoc’h M, Randon J, et al. Involvement of cysteinyl leukotrienes in angiotensin II-induced contraction in isolated aortas from transgenic (mRen-2)27 rats. J Hypertens. 2002;20:263–72.
Schwarz B, Sharma L, Roberts L, Peng X, Bermejo S, Leighton I, et al. Cutting edge: severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J Immunol. 2021;206:329–34.
Archambault AS, Zaid Y, Rakotoarivelo V, Turcotte C, Doré É, Dubuc I, et al. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. Faseb j. 2021;35: e21666.
Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:135–43.
Thompson-Souza GA, Gropillo I, Neves JS. Cysteinyl leukotrienes in eosinophil biology: functional roles and therapeutic perspectives in eosinophilic disorders. Front Med (Lausanne). 2017;4:106.
Scott JP, Peters-Golden M. Antileukotriene agents for the treatment of lung disease. Am J Respir Crit Care Med. 2013;188:538–44.
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71:762–8.
Kim DM, Kim Y, Seo JW, Lee J, Park U, Ha NY, et al. Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19. Cell Rep. 2021;37: 109798.
Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United European Gastroenterol J. 2020;8:509–19.
Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–99.
Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–99.
Sebaldt RJ, Sheller JR, Oates JA, Roberts LJ 2nd, FitzGerald GA. Inhibition of eicosanoid biosynthesis by glucocorticoids in humans. Proc Natl Acad Sci USA. 1990;87:6974–8.
Manso G, Baker AJ, Taylor IK, Fuller RW. In vivo and in vitro effects of glucocorticosteroids on arachidonic acid metabolism and monocyte function in nonasthmatic humans. Eur Respir J. 1992;5:712–6.
Vachier I, Kumlin M, Dahlén SE, Bousquet J, Godard P, Chanez P. High levels of urinary leukotriene E4 excretion in steroid treated patients with severe asthma. Respir Med. 2003;97:1225–9.
Negri J, Early SB, Steinke JW, Borish L. Corticosteroids as inhibitors of cysteinyl leukotriene metabolic and signaling pathways. J Allergy Clin Immunol. 2008;121:1232–7.
Brzezinska-Blaszczyk E, Rdzany RS. Lipoteichoic acids selectively stimulate rat mast cells to cysteinyl leukotriene generation and affect mast cell migration after tumor necrosis factor (TNF)-priming. Immunol Lett. 2007;109:138–44.
Petersen B, Austen KF, Bloch KD, Hotta Y, Ichinose F, Kanaoka Y, et al. Cysteinyl leukotrienes impair hypoxic pulmonary vasoconstriction in endotoxemic mice. Anesthesiology. 2011;115:804–11.
Pollock J, Chalmers JD. The immunomodulatory effects of macrolide antibiotics in respiratory disease. Pulm Pharmacol Ther. 2021;71: 102095.
Dey M, Singh RK. Possible therapeutic potential of cysteinyl leukotriene receptor antagonist montelukast in treatment of SARS-CoV-2-induced COVID-19. Pharmacology. 2021;106:469–76.
Bohnacker S, Hartung F, Henkel F, Quaranta A, Kolmert J, Priller A, et al. Mild COVID-19 imprints a long-term inflammatory eicosanoid- and chemokine memory in monocyte-derived macrophages. Mucosal Immunol. 2022. https://doi.org/10.1038/s41385-021-00482-8.
Palmas F, Clarke J, Colas RA, Gomez EA, Keogh A, Boylan M, et al. Dysregulated plasma lipid mediator profiles in critically ill COVID-19 patients. PLoS One. 2021;16: e0256226.
Camera M, Canzano P, Brambilla M, Rovati GE. Montelukast inhibits platelet activation induced by plasma from COVID-19 patients. Front Pharmacol. 2022;13: 784214.
Bozek A, Winterstein J. Montelukast’s ability to fight COVID-19 infection. J Asthma. 2021;58:1348–9.
Khan AR, Misdary C, Yegya-Raman N, Kim S, Narayanan N, Siddiqui S, et al. Montelukast in hospitalized patients diagnosed with COVID-19. J Asthma. 2021. https://doi.org/10.1080/02770903.2021.1881967:1-7.
Elkin PL, Resendez S, Mullin S, Troen BR, Mammen MJ, Chang S, et al. Leukotriene inhibitors with dexamethasone show promise in the prevention of death in COVID-19 patients with low oxygen saturations. J Clin Transl Sci. 2022;6: e74.
Wilchesky M, Tranmer G, Grad R. The COvid-19 Symptom MOntelukast trial (COSMO). 2020; Identifier NCT04389411. https://clinicaltrials.gov/ct2/show/NCT04389411.
Almeda Ortega J, Bonet Monne S, Salvador Gonzalez B, Mera Cordero F. Efficacy of montelukast in mild-moderate respiratory symptoms in patients with long-COVID-19: (E-SPERANZA). 2021. Identifier NCT04695704. https://clinicaltrials.gov/ct2/show/NCT04695704.
Tavares M, Farraia M, Silva S, Ribeiro AM, Severo M, Paciência I, et al. Impact of montelukast as add on treatment to the novel coronavirus pneumonia (COVID-19): protocol for an investigator-initiated open labeled randomized controlled pragmatic trial. Porto Biomed J. 2021;6: e134.
Acknowledgements
The authors wish to thank the blood donors, medical doctors and nurses from the Service of Immunohemotherapy, CHUSJ, and to the nurses from the Service of Intensive Care Medicine, CHUSJ, for their collaboration in our study.
Funding
This work was supported by a RESEARCH 4 COVID-19 grant (project 519, reference number: 613690173) from FCT—Fundação para a Ciência e a Tecnologia (special support for rapid implementation projects for innovative response solutions to COVID-19 pandemic). CS-P is a recipient of a Ph.D. fellowship from FCT and MedInUP (UI/BD/150816/2020). P-PT was supported by a research contract within the scope of the RIFF-HEART project funded by FEDER via COMPETE, Portugal 2020—Operational Programme for Competitiveness and Internationalization (POCI) (POCI-01-0145-FEDER-032188) and by FCT (PTDC/MEC-CAR/32188/2017). Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Conceptualization: [MR-C]; [TS]; [AA-T]; methodology: [MR-C]; [MT]; [TS]; [CCD]; formal analysis and investigation: [MR-C]; [MR-M]; [PP-T]; [CS-P]; [SM]; [LT-S]; [DP]; [AD]; [GC]; [CCD]; [TS]; writing—original draft: [MR-C]; [MR-M]; writing—review and editing: [MR-C]; [PP-T]; [CS-P]; [DP]; [LT-S]; [CCD]; [MT]; [AS]; [JTG]; [RR-A]; [J-AP]; [AA-T]; [TS]; resources: [MR-C]; [MT]; [AS]; [JTG]; [RR-A]; [J-AP]; [AA-T]; funding acquisition: [AA-T]; supervision: [AA-T]; [TS].
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethics approval
The CHUSJ Health Ethics Committee approved this study [CES 75-16], with project amended specifically for inclusion of subjects with COVID-19, within the scope of a RESEARCH 4 COVID-19 grant from FCT (special support for rapid implementation projects for innovative response solutions to COVID-19 pandemic)].
Consent to participate
All eligible patients provided written informed consent to participate in the study. For ICU patients unable to give consent, this was solicited to their next of kin. These patients provided informed consent retrospectively, where possible. Blood donor volunteers provided oral informed consent.
Consent for publication
All the authors agree to publish this article.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2022_1682_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1. Flowchart indicating the number of severe COVID-19 patients analysed at each time point and the reasons for missing data and patient drop out from the study. (TIF 130 KB)
11_2022_1682_MOESM2_ESM.tif
Supplementary file2 Supplementary Fig. 2. Flowchart indicating the number of critical COVID-19 patients analysed at each time point and the reasons for missing data and patient drop out from the study. (TIF 120 KB)
11_2022_1682_MOESM3_ESM.tif
Supplementary file3 Supplementary Fig. 3. Flowchart indicating the number of critical COVID-19 on VV-ECMO patients analysed at each time point and the reasons for missing data and patient drop out from the study. (TIF 115 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reina-Couto, M., Roboredo-Madeira, M., Pereira-Terra, P. et al. Evaluation of urinary cysteinyl leukotrienes as biomarkers of severity and putative therapeutic targets in COVID-19 patients. Inflamm. Res. 72, 475–491 (2023). https://doi.org/10.1007/s00011-022-01682-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01682-z