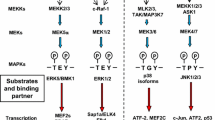
Abstract
Atherosclerosis is a chronic inflammatory disease that is the underlying cause of cardiovascular disease which initiates from endothelial dysfunction from genetic and environmental risk factors, including biomechanical forces: blood flow. Endothelial cells (ECs) lining the inner arterial wall regions exposed to disturbed flow are prone to atherosclerosis development, whereas the straight regions exposed to stable flow are spared from the disease. These flow patterns induce genome- and epigenome-wide changes in gene expression in ECs. Through the sweeping changes in gene expression, disturbed flow reprograms ECs from athero-protected cell types under the stable flow condition to pro-atherogenic cell conditions. The pro-atherogenic changes induced by disturbed flow, in combination with additional risk factors such as hypercholesterolemia, lead to the progression of atherosclerosis. The flow-sensitive genes and proteins are critical in understanding the mechanisms and serve as novel targets for antiatherogenic therapeutics.
Graphical abstract


Modified from Kumar et al., created with BioRender.com. Abbreviations: D-flow, disturbed flow; EC, endothelial cell; LDL, low-density lipoprotein; oxLDL, oxidized LDL; S-flow, stable flow; VSMC, vascular smooth muscle cell


Modified from Cheng et al., 2017, created with BioRender.com Abbreviations: D-flow, disturbed flow; EC, endothelial cell; ECM, extracellular matrix; MWSS, mean wall shear stress

Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- S-flow:
-
Stable flow
- D-flow:
-
Disturbed flow
- EC:
-
Endothelial cells
- LAM:
-
Leukocyte adhesion molecule
- VCAM1:
-
Vascular adhesion molecule 1
- MMP:
-
Metalloproteinases
- BMP4:
-
Bone morphogenic protein
- miR:
-
Micro RNAs
- EndMT:
-
Endothelial to mesenchymal transition
- EndICLT:
-
Endothelial to immune cell-like transition
- ECM:
-
Extracellular matrix
- NO:
-
Nitic oxide
- ROS:
-
Reactive oxygen species
- SMA:
-
Smooth muscle actin
- scATACseq:
-
Single cell assay for transposase-accessible chromatin using sequencing
- EndICLT:
-
Endothelial cell-immune cell-like transition
- LDL:
-
Low-density lipoprotein
- oxLDL:
-
Oxidized LDL
- LRP1:
-
Lipoprotein receptor-related protein 1
- LOX1:
-
Lectin-like oxLDL 1 receptor
- VSMC:
-
Vascular smooth muscle cells
- IFNγ:
-
Interferonγ
- WSS:
-
Wall shear stress
- MWSS:
-
Mean WSS
- Re:
-
Reynold’s number
- TNF:
-
Tumor necrosis factor
- KLF2:
-
Krüppel-like factor 2
- KLF4:
-
Krüppel-like factor 4
- Par1:
-
Protease-activated receptor 1
- Jnk:
-
JUN N-terminal kinase
- ATF2:
-
Activating transcription factor 2
- eNOS:
-
Endothelial nitric oxide synthase
- TGF β:
-
Transforming growth factor β
- FSP1:
-
Fibroblast-specific protein 1
- vWF:
-
Von willlebrand factor
- AV:
-
Aortic valve
- VEC:
-
Valvular EC
- CYR61:
-
Cysteine-rich angiogenic inducer 61
- CTGF:
-
Connective tissue growth factor
- ANKRD1:
-
Ankyrin repeat domain 1
- LATS:
-
Large tumor suppressor
- HIF-1α:
-
Hypoxia inducible factor 1-alpha
- pVHL:
-
Von Hippel-Lindau protein
- PS:
-
Pulsatile shear
- MYH11:
-
Myosin heavy chain
- ABCA1:
-
ATP-binding cassette transporter A1
- IL-10:
-
Interleukin-10
- HBP1:
-
HMG box-transcription protein 1
- MIF:
-
Migration inhibitory factor
- MCP-1:
-
Monocyte chemoattractant protein-1
- ITGA5:
-
Integrin subunits α5
- HAEC:
-
Human aortic endothelial cells
- OS:
-
Oscillatory shear
- LS:
-
Laminar shear
- iMAEC:
-
Immortalized mouse aortic endothelial cells
References
Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018;132:1243–52.
Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–87.
Heo K-S, Fujiwara K, Abe J-i. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J. 2011;75:2722–30.
Davies PF, Polacek DC, Handen JS, et al. A spatial approach to transcriptional profiling: mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999;17:347–51.
Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31.
Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26.
Simmons RD, Kumar S, Jo H. The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches. Arch Biochem Biophys. 2016;591:111–31.
Villa-Roel N, Ryu K, Jo H. Role of Biomechanical stress and mechanosensitive miRNAs in calcific aortic valve disease. In Cardiovascular Calcification and Bone Mineralization, Aikawa, E., Hutcheson, J.D., Eds. Springer International Publishing: Cham, 2020;117–35. https://doi.org/10.1007/978-3-030-46725-8_6pp.
Kumar S, Kim CW, Simmons RD, et al. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014;34:2206–16.
Kumar S, Kim CW, Son DJ, et al. Flow-dependent regulation of genome-wide mRNA and microRNA expression in endothelial cells in vivo. Sci Data. 2014;1:140039.
Simmons RD, Kumar S, Thabet SR, et al. Omics-based approaches to understand mechanosensitive endothelial biology and atherosclerosis. WIREs Syst Biol Med. 2016;8:378–401.
Dunn J, Thabet S, Jo H. Flow-dependent epigenetic DNA methylation in endothelial gene expression and atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1562–9.
Dunn J, Qiu H, Kim S, et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–99.
Ni C-W, Qiu H, Rezvan A, et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73.
Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–508.
Andueza A, Kumar S, Kim J, et al. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. 2020;33:108491.
van Thienen JV, Fledderus JO, Dekker RJ, et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72:231–40.
Kinderlerer AR, Ali F, Johns M, et al. KLF2-dependent, shear stress-induced expression of CD59. J Biol Chem. 2008;283:14636–44.
Hamik A, Lin Z, Kumar A, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–79.
Son DJ, Kumar S, Takabe W, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000.
Nam D, Ni C-W, Rezvan A, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–43.
Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress: role of c-Myc *. J Biol Chem. 2003;278:32994–9.
Jo H, Song H, Mowbray A. Role of NADPH Oxidases in disturbed flow- and BMP4- Induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006;8:1609–19.
Kumar S, Williams D, Sur S, et al. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vasc Pharmacol. 2019;114:76–92.
Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26.
Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3–6.
Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51.
Cybulsky MI, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91.
Dong ZM, Chapman SM, Brown AA, et al. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–52.
Collins RG, Velji R, Guevara NV, et al. P-selectin or intercellular adhesion molecule (Icam)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E–deficient mice. J Exp Med. 2000;191:189–94.
Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–16.
Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–2.
Mudau M, Genis A, Lochner A, et al. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–31.
Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75.
Souilhol C, Harmsen MC, Evans PC, et al. Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc Res. 2018;114:565–77.
Chen P-Y, Qin L, Baeyens N, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–28.
Souilhol C, Serbanovic-Canic J, Fragiadaki M, et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol. 2020;17:52–63.
van Meeteren LA, Ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012;347:177–86.
Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–80.
Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361.
Cho JG, Lee A, Chang W, et al. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol. 2018;9:294.
Stenmark KR, Frid M, Perros F. Endothelial-to-mesenchymal transition. Circulation. 2016;133:1734–7.
Demos C, Williams D, Jo H. Disturbed Flow induces atherosclerosis by annexin A2-mediated integrin activation. Circ Res. 2020;127:1091–3.
Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28:69–75.
Silverstein RL. Febbraio M 2009 CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3.
Zani IA, Stephen SL, Mughal NA, et al. Scavenger receptor structure and function in health and disease. Cells. 2015;4:178–201.
Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–44.
Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–6.
Hansson GK. Cell-mediated immunity in atherosclerosis. Curr Opin Lipidol. 1997;8:301–11.
Gotsman I, Gupta R, Lichtman AH. The influence of the regulatory t lymphocytes on atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2493–5.
Gupta S, Pablo AM, Jiang X-c, et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–61.
Lee YW, Kim PH, Lee WH, et al. Interleukin-4, oxidative stress, vascular inflammation and atherosclerosis. Biomol Ther. 2010;18:135–44.
Majesky MW, Dong XR, Hoglund V, et al. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–9.
Topper JN, Gimbrone MA Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–6.
Tanaka H, Shimizu S, Ohmori F, et al. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–5.
Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011;15:1389–403.
Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam. 2011;2011:263870.
Williams D, Mahmoud M, Liu R, et al. Stable flow-induced expression of KLK10 inhibits endothelial inflammation and atherosclerosis. eLife. 2022;11:e72579.
Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23.
Ghalichi F, Deng X, De Champlain A, et al. Low Reynolds number turbulence modeling of blood flow in arterial stenoses. Biorheology. 1998;35:281–94.
Kinlay S, Grewal J, Manuelin D, et al. Coronary flow velocity and disturbed flow predict adverse clinical outcome after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2002;22:1334–40.
Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117:1082–9.
Hwang J, Ing MH, Salazar A, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–32.
Harrison D, Griendling KK, Landmesser U, et al. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7a–11a.
Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, et al. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–85.
Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–62.
Baek KI, Li R, Jen N, et al. Flow-responsive vascular endothelial growth factor receptor-protein kinase C isoform epsilon signaling mediates glycolytic metabolites for vascular repair. antioxid. Redox Signal. 2018;28:31–43.
Wu D, Huang R-T, Hamanaka RB, et al. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. eLife. 2017;6:e25217.
Li R, Jen N, Wu L, et al. Disturbed flow induces autophagy, but impairs autophagic flux to perturb mitochondrial homeostasis. Antioxid Redox Signal. 2015;23:1207–19.
Cheng C, Helderman F, Tempel D, et al. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis. 2007;195:225–35.
Samijo SK, Willigers JM, Barkhuysen R, et al. Wall shear stress in the human common carotid artery as function of age and gender. Cardiovasc Res. 1998;39:515–22.
Callaghan FM, Grieve SM. Normal patterns of thoracic aortic wall shear stress measured using four-dimensional flow MRI in a large population. Am J Physiol Heart Circ Physiol. 2018;315:H1174–81.
Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–42.
LaBarbera M. Principles of design of fluid transport systems in zoology. Science. 1990;249:992–1000.
Girerd X, London G, Boutouyrie P, et al. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension (Dallas, Tex : 1979). 1996;27:799–803.
Murray CD. The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol. 1926;9:835–41.
Li YH, Reddy AK, Taffet GE, et al. Doppler evaluation of peripheral vascular adaptations to transverse aortic banding in mice. Ultrasound Med Biol. 2003;29:1281–9.
Marano G, Palazzesi S, Vergari A, et al. Protection by shear stress from collar-induced intimal thickening: role of nitric oxide. Arterioscler Thromb Vasc Biol. 1999;19:2609–14.
Ibrahim J, Miyashiro JK, Berk BC. Shear stress is differentially regulated among inbred rat strains. Circ Res. 2003;92:1001–9.
Lee K, Choi M, Yoon J, et al. Spectral waveform analysis of major arteries in conscious dogs by Doppler ultrasonography. Vet Radiol Ultrasound. 2004;45:166–71.
Ross G, White FN, Brown AW, et al. Regional blood flow in the rat. J Appl Physiol. 1966;21:1273–5.
Snow HM, Markos F, O’Regan D, et al. Characteristics of arterial wall shear stress which cause endothelium-dependent vasodilatation in the anaesthetized dog. J Physiol. 2001;531:843–8.
LaDisa JF Jr, Olson LE, Molthen RC, et al. Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am J Physiol Heart Circ Physiol. 2005;288:65–75.
Demos C, Tamargo I, Jo H. Chapter 1 - biomechanical regulation of endothelial function in atherosclerosis. In Biomechanics of Coronary Atherosclerotic Plaque, Ohayon, J., Finet, G., Pettigrew, R.I., Eds. Academic Press: 2021; vol. 4, pp 3–47.
Kwak BR, Bäck M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35(3013–20):20a–20d.
Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615.
Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–24.
Davis ME, Cai H, Drummond GR, et al. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–80.
Gachhui R, Abu-Soud HM, Ghosha DK, et al. Neuronal nitric-oxide synthase interaction with calmodulin-troponin C chimeras. J Biol Chem. 1998;273:5451–4.
Nishida K, Harrison DG, Navas JP, et al. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–6.
De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8.
Dimmeler S, Haendeler J, Nehls M, et al. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–7.
Dimmeler S, Hermann C, Galle J, et al. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–64.
Li J, Billiar TR, Talanian RV, et al. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–24.
Tsao PS, Lewis NP, Alpert S, et al. Exposure to shear stress alters endothelial adhesiveness Role of nitric oxide. Circulation. 1995;92:3513–9.
Rössig L, Haendeler J, Hermann C, et al. Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem. 2000;275:25502–7.
Kim YM, de Vera ME, Watkins SC, et al. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–11.
Sangwung P, Zhou G, Nayak L, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI insight. 2017;2:e91700.
Ghaleb AM, Yang VW. Krüppel-like factor 4 (KLF4): what we currently know. Gene. 2017;611:27–37.
SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–15.
Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36.
Das H, Kumar A, Lin Z, et al. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci USA. 2006;103:6653–8.
Lin Z, Hamik A, Jain R, et al. Kruppel-like factor 2 inhibits protease activated receptor-1 expression and thrombin-mediated endothelial activation. Arterioscler Thromb Vasc Biol. 2006;26:1185–9.
Lingrel JB, Pilcher-Roberts R, Basford JE, et al. Myeloid-specific Krüppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ Res. 2012;110:1294–302.
Sweet DR, Fan L, Hsieh PN, et al. Krüppel-like factors in vascular inflammation: mechanistic insights and therapeutic potential. Frontiers in cardiovascular medicine. 2018;5:6.
Fledderus JO, Boon RA, Volger OL, et al. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–46.
Dekker RJ, van Soest S, Fontijn RD, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood. 2002;100:1689–98.
Turpaev KT. Transcription factor KLF2 and its role in the regulation of inflammatory processes. Biochemistry Biokhimiia. 2020;85:54–67.
Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124:633–41.
Schober A, Weber C. Mechanisms of microRNAs in atherosclerosis. Annu Rev Pathol. 2016;11:583–616.
Mathieu P, Pibarot P, Després JP. Metabolic syndrome: the danger signal in atherosclerosis. Vasc Health Risk Manage. 2006;2:285–302.
Yang Q, Xu J, Ma Q, et al. PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat Commun. 2018;9:4667.
Doddaballapur A, Michalik KM, Manavski Y, et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol. 2015;35:137–45.
Perrotta P, Van der Veken B, Van Der Veken P, et al. Partial inhibition of glycolysis reduces atherogenesis independent of intraplaque neovascularization in mice. Arterioscler Thromb Vasc Biol. 2020;40:1168–81.
Heo KS, Berk BC, Abe J. Disturbed flow-induced endothelial proatherogenic signaling via regulating post-translational modifications and epigenetic events. Antioxid Redox Signal. 2016;25:435–50.
Villarreal G Jr, Zhang Y, Larman HB, et al. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun. 2010;391:984–9.
Endres M, Laufs U, Merz H, et al. Focal expression of intercellular adhesion molecule-1 in the human carotid bifurcation. Stroke. 1997;28:77–82.
Yousef GM, Luo LY, Diamandis EP. Identification of novel human kallikrein-like genes on chromosome 19q13.3–q13.4. Anticancer Res. 1999;19:2843–52.
Diamandis EP, Yousef GM, Clements J, et al. New nomenclature for the human tissue kallikrein gene family. Clin Chem. 2000;46:1855–8.
Wang GL, Jiang B-H, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4.
Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–94.
Greijer A, Van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–14.
Déry M-AC, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem. 2005;37:535–40.
Maxwell PH, Dachs GU, Gleadle JM, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104–9.
Fernandez Esmerats J, Villa-Roel N, Kumar S, et al. Disturbed flow increases UBE2C (Ubiquitin E2 Ligase C) via loss of miR-483-3p, inducing aortic valve calcification by the pVHL (von Hippel-Lindau protein) and HIF-1α (hypoxia-inducible factor-1α) pathway in endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39:467–81.
Feng S, Bowden N, Fragiadaki M, et al. Mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arterioscler Thromb Vasc Biol. 2017;37:2087–101.
Akhtar S, Hartmann P, Karshovska E, et al. Endothelial hypoxia-inducible factor-1α promotes atherosclerosis and monocyte recruitment by upregulating microRNA-19a. Hypertension (Dallas, Tex : 1979). 2015;66:1220–6.
Salim MT, Villa-Roel N, Vogel B, et al. HIF1A inhibitor PX-478 reduces pathological stretch-induced calcification and collagen turnover in aortic valve. Front Cardiovasc Med. 2022;9:1002067.
Demos C, Johnson J, Andueza A, et al. Sox13 is a novel flow-sensitive transcription factor that prevents inflammation by repressing chemokine expression in endothelial cells. Front Cardiovasc Med. 2022;9:979745.
Wang K-C, Garmire LX, Young A, et al. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A. 2010;107:3234–9.
Iaconetti C, De Rosa S, Polimeni A, et al. Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc Res. 2015;107:522–33.
Chen K, Fan W, Wang X, et al. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun. 2012;427:138–42.
Zhang N, Lei J, Lei H, et al. MicroRNA-101 overexpression by IL-6 and TNF-α inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression. Exp Cell Res. 2015;336:33–42.
Qin X, Wang X, Wang Y, et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:3240–4.
Ren Z-Q, Liu N, Zhao K. Micro RNA-19a suppresses IL-10 in peripheral B cells from patients with atherosclerosis. Cytokine. 2016;86:86–91.
Chen H, Li X, Liu S, et al. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Sci Rep. 2017;7:12089.
Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–87.
Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–3.
Wang W-L, Chen L-J, Wei S-Y, et al. Mechanoresponsive Smad5 enhances MiR-487a processing to promote vascular endothelial proliferation in response to disturbed flow. Front Cell Dev Biol. 2021;9:647714.
Lee S, Jue M, Cho M, et al. Label-free atherosclerosis diagnosis through a blood drop of apolipoprotein E knockout mouse model using surface-enhanced Raman spectroscopy validated by machine learning algorithm. Bioeng Transl Med. 2023;8:e10529.
Acknowledgements
This study is supported by the National Institutes of Health 5T32HL007745-28 (KB).
Funding
National Institutes of Health, 5T32HL007745-28, Kyung In Baek
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human Subjects/Informed Consent Statement
No human studies were carried out by the authors for this article.
Animal Studies
No animal studies were carried out by the authors for this article.
Additional information
Associate Editor Judith Sluimer oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baek, K.I., Ryu, K. Role of Flow-Sensitive Endothelial Genes in Atherosclerosis and Antiatherogenic Therapeutics Development. J. of Cardiovasc. Trans. Res. (2023). https://doi.org/10.1007/s12265-023-10463-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-023-10463-w