Formation of anhydrides from acid halides and carboxylates
Description: Addition of a carboxylic acid to an acid chloride results in the anhydride, displacing the chloride ion.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples
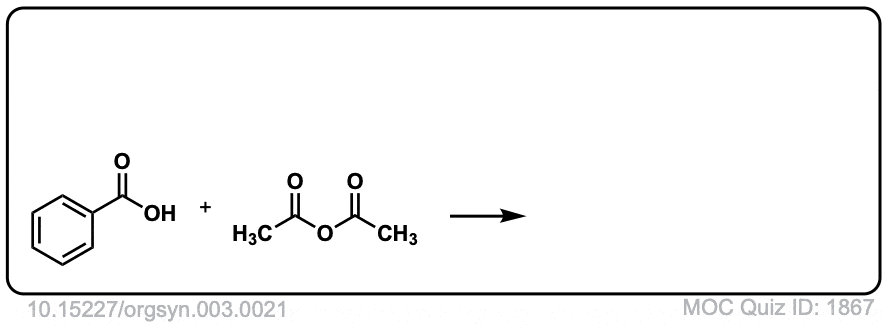
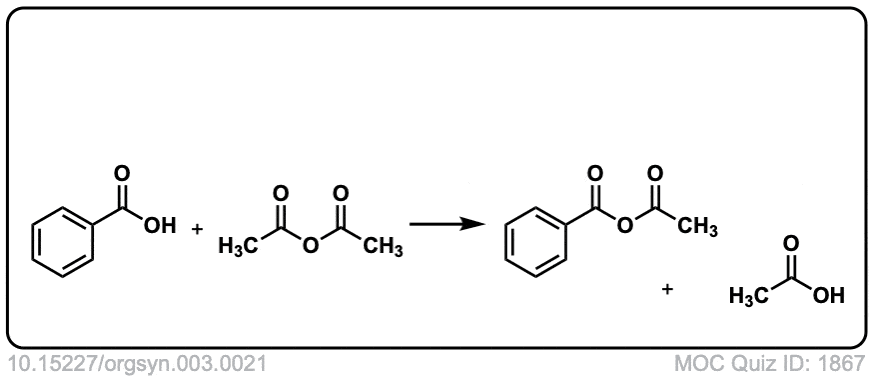
Org. Synth. 1923, 3, 21
DOI Link: 10.15227/orgsyn.003.0021
 Click to Flip
Click to Flip
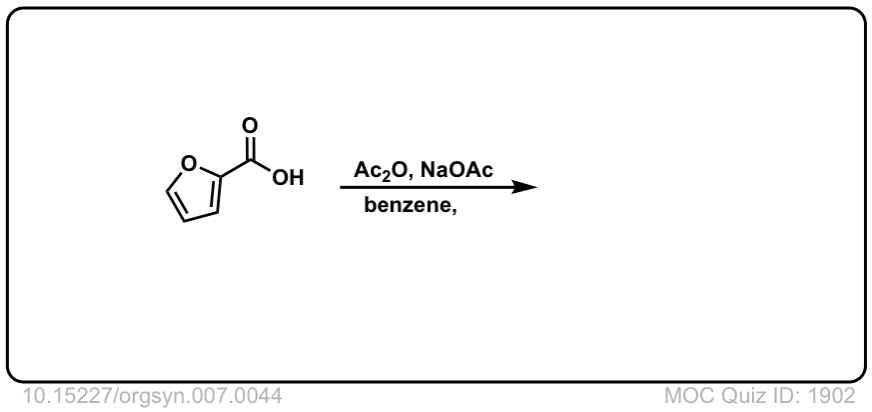
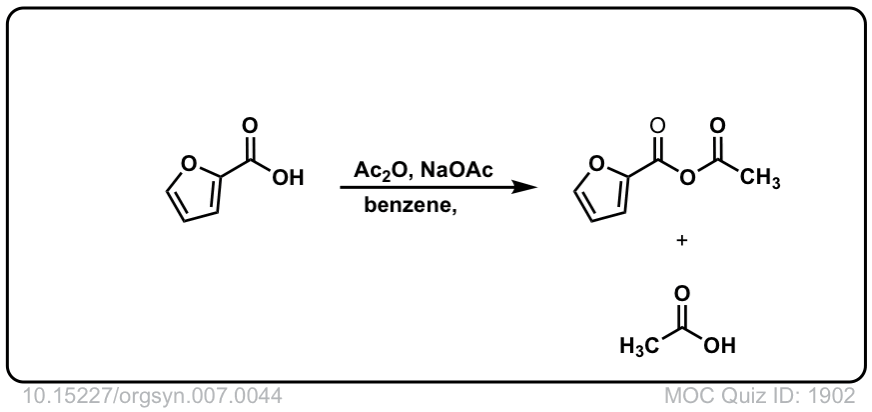
Org. Synth. 1927, 7, 44
DOI Link: 10.15227/orgsyn.007.0044
 Click to Flip
Click to Flip
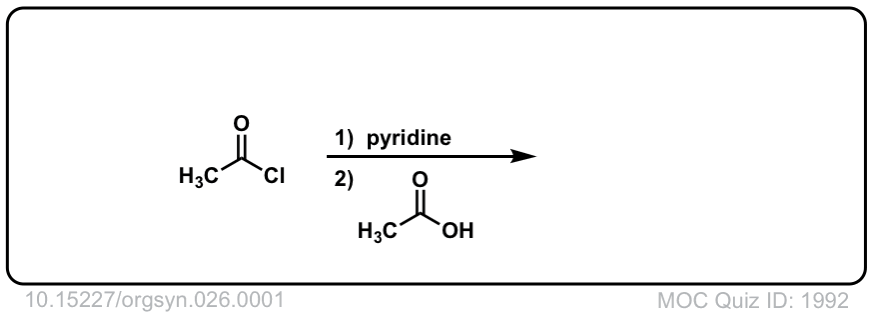
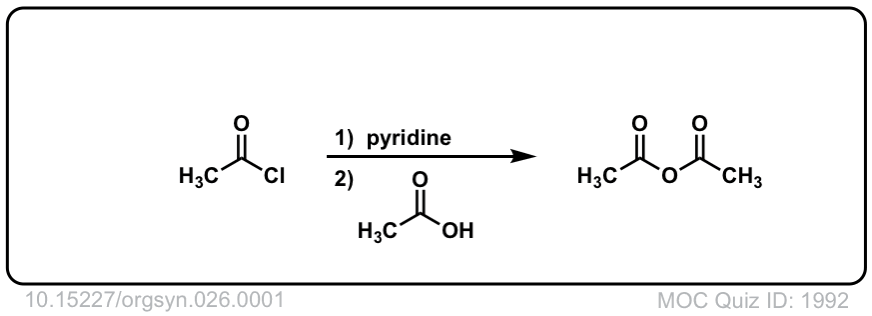
Org. Synth. 1946, 26, 1
DOI Link: 10.15227/orgsyn.026.0001
 Click to Flip
Click to Flip
Hi. Can also an ester with a carboxylic acid and pyridine being used to make an anhydride?
That’s going to be tough, because the pyridine will be a nucleophile and can react with an anhydride.
Hi. Can also an ester with a carboxylic acid being used to make an anhydride?
No, the ester has to be hydrolyzed to a carboxylic acid first.
Hi, the reaction of acid halides reacting with carboxylic acid in pyridine to form anhydride is not mentioned. Just curious why. Thanks.
That would also be a valid method; using pyridine as the base to form the carboxylate from the carboxylic acid. One is spoiled for choice in terms of bases to use!
There’s this other reaction I learned in class for the formation of anhydrides–you can react two moles of carboxylic acid, with H2SO4 and heat, to form an anhydride. (We were not clearly taught the mechanism, however–correct me if I am wrong, but I assume that one of the OH groups nab the H off the other OH group and leave as H2O, so that an anhydride is formed.)
However, I have also read in a textbook (Solomons and Fryhle, specificially) that an anhydride can be formed using only heat, but only when the cyclic anhydride forms a 5- or 6-membered ring. What’s the significance of the H2SO4 from the first method, then?
In any case, using acyl halides and a carboxylate would be more effective, as mentioned above. But how so? I mean, does it give a faster reaction rate? Or is it more “efficient” (more yield)? Or a mixture of both?
Sorry if I’m asking too many questions, hehe. Curiosity killed the cat (but satisfaction brought it back).