Abstract
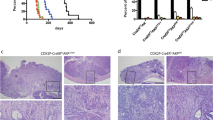
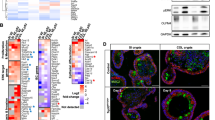
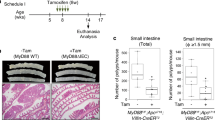
Human colorectal cancers are known to possess multiple mutations, though how these mutations interact in tumor development and progression has not been fully investigated. We have previously described the FCPIK3ca* murine colon cancer model, which expresses a constitutively activated phosphoinositide-3 kinase (PI3K) in the intestinal epithelium. The expression of this dominantly active form of PI3K results in hyperplasia and invasive mucinous adenocarcinomas. These cancers form via a non-canonical mechanism of tumor initiation that is mediated through activation of PI3K and not through aberrations in WNT signaling. Since the Adenomatous Polyposis Coli (APC) gene is mutated in the majority of human colon cancers and often occurs simultaneously with PIK3CA mutations, we sought to better understand the interaction between APC and PIK3CA mutations in the mammalian intestine. In this study, we have generated mice in which the expression of a constitutively active PI3K and the loss of APC occur simultaneously in the distal small intestine and colon. Here, we demonstrate that expression of a dominant active PI3K synergizes with loss of APC activity resulting in a dramatic change in tumor multiplicity, size, morphology and invasiveness. Activation of the PI3K pathway is not able to directly activate WNT signaling through the nuclear localization of CTNNB1 (β-catenin) in the absence of aberrant WNT signaling. Alterations at the transcriptional level, including increased CCND1, may be the etiology of synergy between these activated pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
American Cancer Society Cancer Facts & Figures 2012. American Cancer Society, Atlanta, 2012.
Cancer Genome Atlas Network, Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337.
Kinzler KW, Vogelstein B . Colorectal tumors. In: Vogelstein B, Kinzler KW, The Genetic Basis of Human Cancer 2nd ed McGraw-Hill, New York, 2002. 583–612.
Goss KH, Groden J . Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol 2000; 18: 1967–1979.
Clevers H, Nusse R . Wnt/β-catenin signaling and disease. Cell 2012; 149: 1192–1205.
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992; 256: 668–670.
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304: 554.
Markowitz SD, Bertagnolli MM . Molecular basis of colorectal cancer. N Engl J Med 2009; 361: 2449–2460.
Zhao L, Vogt PK . Hot-spot mutations in p110α of phosphatidylinositol 3-kinase (PI3K):differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle 2010; 9: 596–600.
Leystra AA, Deming DA, Zahm CD, Farhoud M, Olson TJ, Hadac JN et al. Mice expressing activated PI3K rapidly develop advanced colon cancer. Cancer Res 2012; 72: 2931–2936.
Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia 2008; 10: 534–541.
Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C . Insulin and IGF-1 stimulate the beta-catenin pathway through two signaling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 2001; 20: 252–259.
Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 2001; 276: 17479–17483.
Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res 2005; 97: 144–151.
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007. 11221–11229.
Ding VW, Chen RH, McCormick F . Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem 2000; 275: 32475–32481.
Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC et al. Phosphatidylinositol 3-kinase signaling does not activate the Wnt cascade. J Biol Chem 2009; 285: 35308–35313.
Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B . Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systemic review with meta-analysis. Eur J Cancer 2009; 45: 1890–1896.
Bacher JW, Abdel Megid WM, Kent-First MG, Halberg RB . Use of mononucleotide repeat markers for detection of microsatellite instability in mouse tumors. Mol Carcinog 2005; 44: 285–292.
Tanwar PS, Zhang L, Roberts DJ, Teixeira JM . Stromal deletion of APC tumor suppressor in mice triggers development of endometrial cancers. Cancer Res 2011; 71: 1584–1596.
Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci USA 2010; 107: 1565–1570.
Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 2012; 367: 1596–1651.
Whitehall VL, Rickman C, Bond CE, Ramsnes I, Greco SA, Umapathy A et al. Oncogenic PIK3CA mutations in colorectal cancers and polyps. Int J Cancer 2012; 131: 813–820.
Velho S, Moutinho C, Cirnes L, Albuquerque C, Hamelin R, Schmitt F et al. BRAF, KRAS, and PIK3CA mutations in colorectal serrrated polyps and cancer: primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer 2008; 8: 255–260.
Armaghany T, Wilson JD, Chu Q, Mills G . Genetic alterations in colorectal cancer. Gastrointest Cancer Res 2012; 5: 19–27.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116: 1175–1186.
Frame S, Cohen P, Biondi RM . A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 2001; 7: 1321–1327.
Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A . Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. J Biol Chem 2000; 275: 34399–34406.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789.
Saam JR, Gordon JI . Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem 1999; 274: 38071–38082.
Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009; 139: 573–586.
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248–5257.
Acknowledgements
We thank Ella Ward and Jane Weeks in Experimental Pathology at the UW Carbone Cancer Center for their technical assistance. This project was supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology through A Young Investigator Award (DAD); the National Cancer Institute of the US National Institutes of Health through T32 CA009614 (DAD), P50 CA095103 (Gastrointestinal Specialized Program of Research Excellence Grant, Vanderbilt Ingram Cancer Center), R01 CA123438 (RBH), P30 CA014520 (Core Grant, University of Wisconsin Carbone Cancer Center); and start-up funds (RBH) from the UW Division of Gastroenterology and Hepatology, the UW Department of Medicine, and the UW School of Medicine and Public Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Jamey Weichert is the founder of Cellectar, Inc. (Madison, WI), which holds the licensing rights to the CLR1404 technology, and therefore has a financial interest in this agent.
Additional information
Author contributions
DAD, AAL and RBH designed, performed and analyzed experiments, and wrote the manuscript. LN, CS, MM, DA and JB performed and analyzed experiments. MKW, LC and JW analyzed experiments. All authors discussed results and edited the manuscript.
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Deming, D., Leystra, A., Nettekoven, L. et al. PIK3CA and APC mutations are synergistic in the development of intestinal cancers. Oncogene 33, 2245–2254 (2014). https://doi.org/10.1038/onc.2013.167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.167
Keywords
This article is cited by
-
PIK3CA mutation is a favorable prognostic factor in esophageal cancer: molecular profile by next-generation sequencing using surgically resected formalin-fixed, paraffin-embedded tissue
BMC Cancer (2018)
-
A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression
Nature Protocols (2018)
-
A proteomic landscape of diffuse-type gastric cancer
Nature Communications (2018)
-
The mathematics of cancer: integrating quantitative models
Nature Reviews Cancer (2015)
-
Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology
Oncogene (2014)