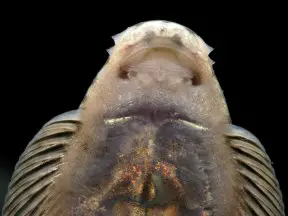
Pseudogastromyzon myersi
Classification
Balitoridae
Distribution
The type locality is given simply as “Hong Kong” and the species is now known to be widespread throughout less-developed parts of the territory now referred to as ‘Hong Kong Special Administrative Region’ plus the neighbouring province of Guangdong, southeastern China. All of those collected for the aquarium trade originate from areas around the city of Guangzhou (Z. Hang, pers. comm.)
Habitat
Mostly restricted to shallow, fast-flowing, highly-oxygenated headwaters/minor tributaries characterised by stretches of riffles and runs broken up by pools or cascades in some cases. Substrates are normally composed of smaller rocks, sand and gravel with jumbles of boulders, and while riparian/stream-side vegetation and patches of submerged leaf litter are common features aquatic plants aren’t usually present. Information regarding sympatric species is lacking but it certainly occurs alongside Liniparhomaloptera disparis, Schistura fasciolata and Rhinogobius duospilus on a frequent basis.
The most favourable habitats contain clear, oxygen-saturated water which, allied with the sun, supports the development of a rich biofilm carpeting submerged surfaces. Hong Kong and Guangzhou have a humid, subtropical, monsoon-influenced climate, and during periods of high rainfall some streams may be temporarily turbid due to suspended material dislodged by increased (sometimes torrential) flow rate and water depth. In Hong Kong at least the water is often slightly acidic, nutrient poor and of low conductivity (Yang and Dudgeon, 2010).
Maximum Standard Length
50 – 60 mm.
Aquarium SizeTop ↑
Minimum base dimensions of 75 cm x 30 cm or equivalent are recommended.
Maintenance
Most importantly the water must be clean and well-oxygenated so we suggest the use of an over-sized filter as a minimum requirement. Although torrential conditions are unnecessary turnover should ideally be in excess of 10 times per hour so supplementary powerheads and airstones can be used to achieve the desired flow/oxygenation.
Base substrate can either be of gravel, sand or a mixture of both to which should be added a layer of water-worn rocks and pebbles of varying sizes. Aged driftwood is also suitable but avoid new pieces since these usually leach tannins which discolour the water and reduce the effectiveness of artificial lighting. The latter must be strong to promote the growth of algae and associated micro-organisms habitually grazes biofilm, and some enthusiasts even maintain an open filter sponge in the tank to provide an extra food source.
Although not a feature of the natural habitat aquatic plants can be used with hardier species such as Microsorum pteropus (Java fern), Crinum and Anubias spp. likely to fare best. The latter are particularly useful as their leaves tend to attract algal growth and provide additional cover for the fish. Since they require stable water conditions and graze biofilm Pseudogastromyzon spp. should never be added to immature set-ups and a tightly-fitting cover is necessary since they are able to climb glass to an extent. While regular partial water changes are essential the rest of the tank needn’t be kept too clean and algae should ideally be allowed to grow on all surfaces except the viewing pane.
Water Conditions
Temperature: Annual air temperatures across its native range average between 61 – 83.7°F/16.1 – 28.7℃. For general aquarium care a water temperature value of 66 – 75°F/18.9 – 23.9°C is therefore recommended, though it can withstand warmer conditions provided dissolved oxygen levels are maintained.
pH: 6.0 – 7.5
Hardness: 1 – 12°H
Diet
Pseudogastromyzon spp. are specialised biofilm grazers feeding chiefly on filamentous Cyanobacteria and stalked diatoms with some microinvertebrates consumed as well, while ‘long’ filamentous or adherent algae tend not to be ingested in great quantities. In captivity some sinking dried foods may be accepted but regular meals of live or frozen Daphnia, Artemia, bloodworm, etc. are essential for the maintenance of good health and it’s highly preferable if the tank contains rock and other solid surfaces with growths of algae and other aufwuchs.
If unable to grow sufficient algae in the main tank or you have a community containing numerous herbivorous fishes which consume what’s available quickly it may be necessary to maintain a separate tank in which to grow algae on rocks and switch them with those in the main tank on a cyclical basis. Such a ‘nursery‘ doesn’t have to be very large, requires only strong lighting and in sunny climates can be kept outdoors.
Balitorids are often seen on sale in an emaciated state which can be difficult to correct. A good dealer will have done something about this prior to sale but if you decide to take a chance with severely weakened specimens they’ll initially require a continual, easily-obtainable source of suitable foods in the absence of competitors if they’re to recover.
Behaviour and CompatibilityTop ↑
Not a particularly aggressive fish although its environmental requirements somewhat limit the choice of suitable tankmates, and it tends to react belligerently to similarly-shaped fishes, especially other balitorids. Species inhabiting similar environments in nature and potentially available include various representatives of Barilius, Discherodontus, Garra, Devario, Rasbora, Rhinogobius, Sicyopterus and Stiphodon plus catfishes like Glyptothorax, Akysis and Hara spp.. Research your choices before purchase in order to be sure and please note that although sometimes sold as such this species makes a poor companion for goldfish.
Many loaches from the family Nemacheilidae and most from Balitoridae are also suitable although squabbles may occur with members of the latter group in particular. These are normally harmless but smaller, less-dominant Gastromyzon or Hypergastromyzon species can suffer from excessive harassment and are not recommended tankmates. More appropriate are members of larger, more robust genera such as Beaufortia, Sewellia or Sinogastromyzon.
It exists in loose aggregations in nature so buy a group of six or more to see its most interesting behaviour. Males are bolder than females and if a group containing both sexes is present will form small territories in all parts of the tank, with the alpha individual(s) occupying the most favourable feeding spots. These are defended against conspecific males and most similar-looking species.
Sexual Dimorphism
Sexually mature females are the larger and fuller-bodied sex, and tend to have less-contrasting markings on the body than males. The latter also develop tubercules on the head once they reach sexual maturity which are more pronounced in dominant individuals.
Reproduction
P. myersi has been bred in aquaria on numerous occasions and unlike some relatives e.g. Sinogastromyzon spp. exhibits a breeding strategy which suggests a non-migratory existence in nature. In a community set-up small numbers of fry often survive but in order to maximise yield you may wish to set up a dedicated breeding tank. It’s worth noting that in nature this species breeds once per year in June/July.
Perhaps the most important aspect in a controlled breeding attempt is provision of a suitable, mature substrate. A medium grade of rounded river gravel is best as the many nooks and crannies created provide refuge for the fry until large enough to escape predation. The organic detritus that tends to collect in this type of substrate also provides a food source for micro-organisms which in turn are eaten by fry. Other décor can consist of some smooth, algae-covered rocks for the adults plus an air-powered, sponge-type filter to provide oxygenation and an additional grazing surface. This type of filter has an added advantage in that eggs/fry won’t be drawn into it.
A group of adults can then be introduced and males will be observed sparring to establish dominance, typified by the fish attempting to overwhelm one another using charging attacks and a suppression technique which loach enthusiasts sometimes refer to as ‘topping’ in which one individual tries to force its way on top of another. They also intensify in colour somewhat. Once a male has successfully established dominance the development of breeding tubercules on the head ensues and he will attempt to entice passing females into his territory by circling and displaying at her. This behaviour can be quite persistent and may also involve following the female around the tank.
Eggs are laid within a ‘nest’ concealed within the substrate and constructed by the male. Sexually mature, dominant individuals will be observed inserting the rear portion of the body and caudal fin into the substrate adjacent to a chosen rock or pebble and clearing a small space using vigorous thrusting and beating movements. This is usually done from a position on the rock itself, presumably to aid leverage and provide a better vantage point. Several pits may be excavated but only one used during each spawn.
A receptive female will enter the nest and lay a batch of eggs before the male moves in to fertilise them, this cycle being repeated several times during a single spawning event. Once the female is spent of eggs the male covers the nest with a layer of substrate.
There is no additional broodcare and the eggs and fry are left to develop by themselves, so if using a dedicated breeding aquarium the adults can now be removed. The length of the incubation period is likely influenced by temperature but reports vary from 4 to 14 days. Once hatched the fry remain within the substrate until free-swimming and usually emerge at a size around 10 mm TL. They begin to graze immediately and can reach 20 – 25 mm TL in a month or so.
NotesTop ↑
This species is traded under various vernacular names including ‘Chinese hillstream loach’, ‘Chinese butterfly plec’ and ‘Hong Kong Otocinclus. It’s the commonest member of the genus in the hobby but has been frequently misidentified as the congener P. cheni in the past with the two often said to be indistinguishable in terms of colour pattern. Some sources additionally suggest the sole defining external character to be that in P. cheni the distance between the respective origins of pectoral and pelvic fins is greater than that between the pelvic fin origin and anus, whereas in P. myersi these distances are equal.
In reality however P. cheni is not collected nor exported for the hobby, and though it may indeed resemble P. myersi its live colour pattern remains unconfirmed at present (Freyhof, 2004). Moreover, it was described from the Ting River (Tingjiang) in Changting County, Fujian province, southeastern China, a drainage forming the upper course of the Han River (Hanjiang) and located several hundred kilometres away from areas in which commercial fish collections occur (see ‘origin’). All Pseudogastromyzon in the aquarium trade exhibiting a dorsal fin with yellow base pigmentation and a red distal band should thus be considered P. myersi and are exclusively collected in the vicinity of Guangzhou (Z. Hang, pers. comm.).
The fact that no diagnostic key to the genus exists in English at time of writing has only complicated matters further, and P. myersi is also quite variable in terms of colour pattern. Within single shipments it’s normally possible to observe individuals both with and without a red distal band in the dorsal fin or with a greater/lesser amount of dark markings on the body, and the fish can also appear lighter or darker depending on mood or environment. In all likelihood this is related to natural variation both within and between populations as seen in some related genera such as Beaufortia and Gastromyzon.
Like all balitorids Pseudogastromyzon spp. have specialised morphology adapted to life in fast-flowing water; the paired fins are orientated horizontally, head and body flattened. These features form a powerful sucking cup which allows the fish to cling tightly to solid surfaces. The ability to swim in open water is greatly reduced and they instead ‘crawl’ their way over and under rocks.
The family Balitoridae is widely-distributed across most of Eurasia with the Indian subcontinent, Southeast Asia and China representing particular centres of species diversity. It was first proposed as a genetically distinct grouping in 2006, and according to current thinking contains over 32 genera of which the most well-known in the aquarium trade are Annamia, Beaufortia, Gastromyzon, Homaloptera, Liniparhomaloptera, Pseudogastromyzon, Sewellia, Sinogastromyzon and Vanmanenia. These were previously considered members of the family Balitoridae, subfamily Balitorinae, but phylogenetic studies have revealed that though closely related Balitorid and Nemacheilid loaches did not evolve from the same common ancestor and represent separate genetic lineages. As it now stands the Nemacheilidae (formerly Balitoridae, subfamily Nemacheilinae) numbers over 30 genera including some popular aquarium subjects such as Aborichthys, Acanthocobitis, Barbatula, Barbucca, Longischistura, Mesonemacheilus, Nemacheilus, Physoschistura, Schistura and Yunnanilus.
References
- Freyhof, J. 2004 - DATZ 2004(11): 70-72
Flossensauger aus China. - Freyhof, J. 2004 - DATZ 2004(11): 70-72
Flossensauger aus China. - Tan, H. H. 2006 - Natural History Publications (Borneo), Kota Kinabalu. 245 p.
The Borneo suckers. Revision of the Torrent Loaches of Borneo (Balitoridae: Gastromyzon, Neogastromyzon). - Tang, Q., H. Liu, R. Mayden and B. Xiong. 2006 - Molecular Phylogenetics and Evolution 39(2): 347-357
Comparison of evolutionary rates in the mitochondrial DNA cytochrome b gene and control region and their implications for phylogeny of the Cobitoidea (Teleostei: Cypriniformes). - Tang, W.-Q. and Y.-Y. Chen. 2000 - Journal of Shanghai Fisheries University 9(1): 1-9
Study on taxonomy of Homalopteridae. - Yang, G. Y. and D. Dudgeon. 2009 - Freshwater Biology 54: 1960–1976
Seasonal and inter-stream variations in the population dynamics, growth and secondary production of an algivorous fish (Pseudogastromyzon myersi: Balitoridae) in monsoonal Hong Kong. - Yang, G. Y. and D. Dudgeon. 2010 - Freshwater Biology 55: 411–423
Response of grazing impacts of an algivorous fish (Pseudogastromyzon myersi: Balitoridae) to seasonal disturbance in Hong Kong streams.