Published online Feb 21, 2023. doi: 10.3748/wjg.v29.i7.1139
Peer-review started: September 21, 2022
First decision: October 18, 2022
Revised: November 2, 2022
Accepted: February 9, 2023
Article in press: February 9, 2023
Published online: February 21, 2023
This review summarizes the evidence about telemonitoring in patients with inflammatory bowel disease (IBD). To give an overview of the advances per
Core Tip: In this review we focus on the advances performed in telemonitoring of patients with inflammatory bowel disease, taking into consideration the elements which enabled its use and how technological achievements led to other barriers for its full implementation. We detail the impact of telemonitoring on health outcomes and its cost-effectiveness. We also describe the advances on new patient-reported outcome measures, home-based tests and wearables which improve the ability to manage new patients´ profiles remotely. However, during the pandemic, e-mail and telephone still represented the main resources used. Then, we describe the emerging barriers which explained the limited application of mature telemonitoring programs.
- Citation: Del Hoyo J, Millán M, Garrido-Marín A, Aguas M. Are we ready for telemonitoring inflammatory bowel disease? A review of advances, enablers, and barriers. World J Gastroenterol 2023; 29(7): 1139-1156
- URL: https://www.wjgnet.com/1007-9327/full/v29/i7/1139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i7.1139
Inflammatory bowel disease (IBD) is a group of disorders characterized by the chronic and recurrent inflammation of different segments of the gastrointestinal tract, which usually associates extraintestinal manifestations and complications due to sustained activity. Unlike other chronic pathologies, IBD mainly affects young individuals in their optimal period of personal and professional development. As such, IBD is related to high levels of school absenteeism and work disability[1], interference with social activities, and impaired health-related quality of life (QoL)[2]. Therefore, IBD has a significant medical, social, and financial impact, further increased by the global increase in its incidence and prevalence in recent years[3].
It is suggested that the “treat-to-target” strategy leads to better outcomes[4]. However, in the conventional management of IBD, scheduled outpatient visits show difficulties to address the disease evolution in each patient, with frequent discrepancies between medical practice and guideline recommendations. Furthermore, patients have little involvement in decision-making, and nearly 50% of adults[5] and an even higher percentage of adolescents with IBD[6,7] are nonadherent to treatment. All these factors prevent the effectiveness of traditional interventions in disease control and increase health expenses[8], especially considering that patients with IBD use health care resources more often than patients with other conditions[9].
Nowadays, health systems are facing financial problems, and telemedicine has been proposed as an alternative to provide an efficient and equitable use of health resources. Information and commu
Telemedicine has been successfully used in other chronic diseases such as congestive heart failure[12,13], diabetes mellitus[14,15] or chronic obstructive pulmonary disease[16,17] and showed excellent acceptance by patients, improvement in health related QoL and a reduction in hospitalizations[12,16,18]. Owing to these positive results, telemedicine systems have been evaluated in patients with IBD, especially in mild to moderate ulcerative colitis (UC)[19,20]. Telemedicine in IBD started with the adaptation of telemonitoring programs previously used in other chronic pathologies[21], but these were subsequently replaced by web and m-health systems, which represented more attractive options to maintain patients adherence to remote follow-up.
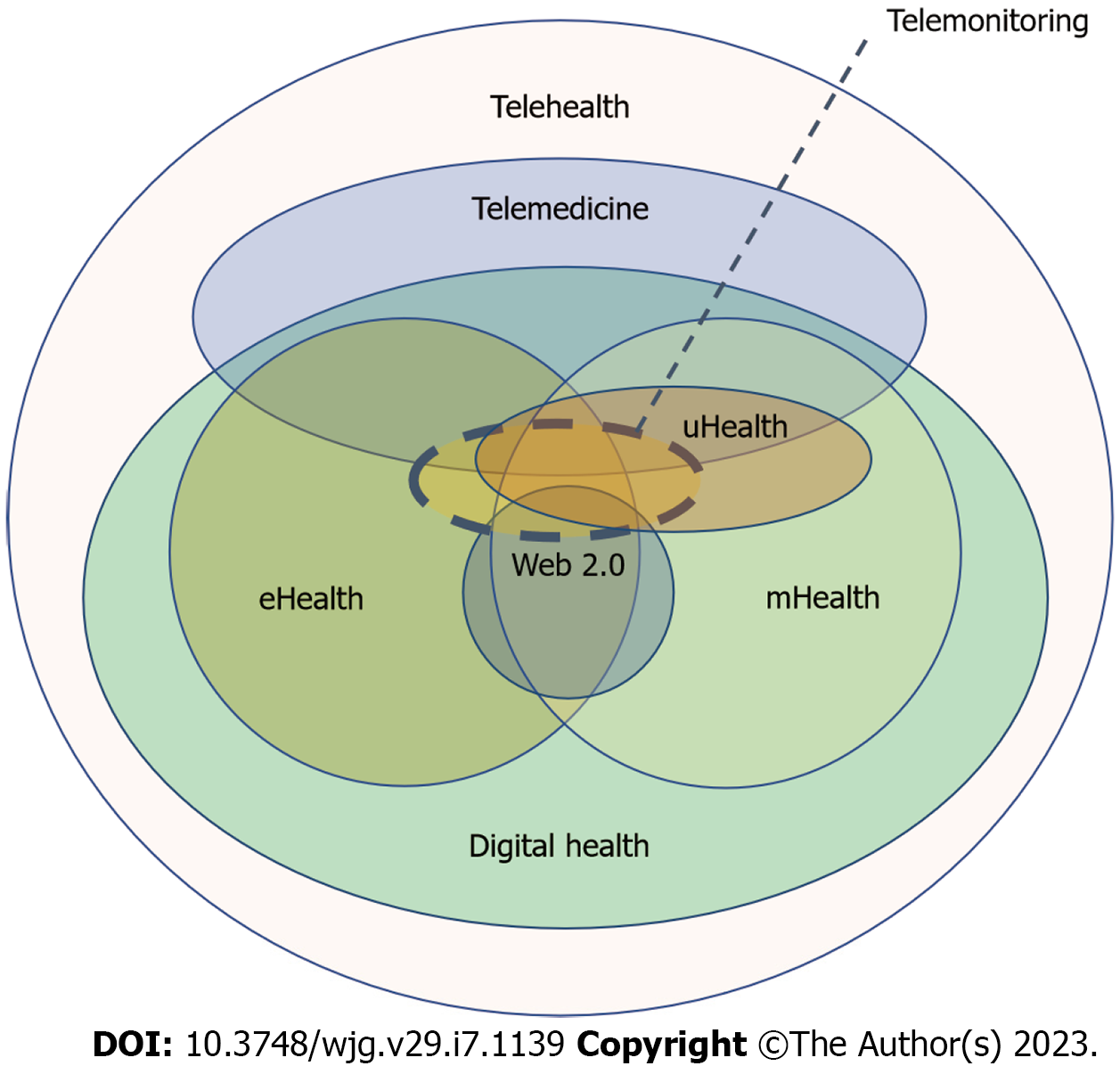
Telemonitoring is the main form of telemedicine in IBD. It is based on the provision of health services at a distance, related to diagnosis, treatment, follow-up or education. It is characterized by the str
The development of more sophisticated telemonitoring programs and point of care (PoC) testing during recent years provided additional value to remote follow-up in the IBD context, improving the ability to cover different patients’ profiles more objectively. These advances gained special interest after the advent of coronavirus disease 2019 (COVID-19) outbreak, as distance management offered new elements to overcome healthcare challenges posed during the pandemic[28]. However, telemedicine was represented mainly by telephone and e-mail, previously available in many centres[29,30], but the development of mature telemedicine programs integrated with electronic health records were still the exception, due to a series of remaining barriers.
In this review we focus on the advances performed in telemonitoring of patients with IBD, taking into consideration the elements which enabled its use and how technological achievements led to other barriers for its full implementation. The search strategy is detailed in the Supplementary material.
There is a wide heterogeneity of telemedicine programs considering the different types of technological resources used, the different diseases and populations in which they are applied, and the objectives pursued with their use. The variability in the quality and design of the published studies (randomized clinical trials, before-and-after studies, qualitative studies, etc.) can partially explain the variable results obtained. Furthermore, in some studies these systems are part of wider interventions, rendering the comparability between programs even more difficult. These factors limit the quality of evidence regarding the efficacy of telemedicine to improve outcomes in chronic diseases.
Despite this, ICTs have been used in a wide range of pathologies, with improvement of patients´ empowerment and with good acceptance[31]. With the aim of giving response to the rise in chronic diseases and multimorbidity worldwide, different projects in Europe have studied the use of telehealth, mainly in diabetes mellitus, cardiovascular diseases, depression, and chronic obstructive pulmonary disease (COPD).
There is moderate evidence about the efficacy of telehealth systems in the improvement of glycaemic control, mainly in terms of HbA1c in patients with type 2 diabetes mellitus. Active telemonitoring including providers´ feedback, as well as tele-education have shown positive results compared with usual practice[14]. In fact, the highest impact was seen in the combination of telemonitoring and tele-education for both patients and providers, allowing for shared decision-making[15].
In patients with heart failure, telemonitoring has been shown to reduce global mortality and hospitalizations compared to usual care[12]. Many telemonitoring systems were part of multidisciplinary programs managed by specialized nurses and incorporated tele-education and action plans before hospital discharge[13]. Interactive monitoring with healthcare providers has also shown to improve blood pressure in hypertensive patients, weight control and lipidic profile[32]. Video consultation and biosensors are especially useful in cardiovascular diseases, with reduced costs compared with other pathologies[33].
Most digital resources used in the mental health context refer to the application of cognitive behavioural therapy (CBT) in patients with depression. Both traditional and e-Health CBT are effective[34], but its use with telemedicine programs could offer additional advantages, such as better accessibility. However, many studies with psychotherapies show a high rate of nonadherence to follow-up[35]. Similarly, in patients with IBD one clinical trial showed a significant improvement in QoL after 12 wk of self-administered computerized CBT, but this outcome was not maintained at 6 mo, with a high rate of dropouts[36].
In patients with asthma, the use of multiplatform programs combining tele-education, telemonitoring and individualized action plans reduced hospitalizations compared with traditional care, mainly in more severe patients[18]. In patients with COPD, the use of telemedicine also reduces hospitalizations, but without an improvement of global mortality[16]. With the development of mHealth, the use of SMS combined with telephone support is associated with an improvement in respiratory function and QoL in patients with asthma[32], but telemonitoring in COPD has not demonstrated any improvement in these outcomes[17,37]. Telemonitoring of patients with COPD is more expensive due to associated multimorbidity[33].
The use of telemedicine in digestive diseases is more limited, and most studies focused on IBD and irritable bowel syndrome. Unlike other chronic diseases such as diabetes mellitus, IBD implies the consideration of many clinical, biological, endoscopic, and even histologic variables to reach disease control. However, early detection of complications usually requires invasive tests in IBD. The absence of validated tools with adequate cost and accuracy to measure disease activity at a distance have represented an important limitation.
Most studies about telemedicine in IBD have emerged in the last decade. The development of mHealth, the validation of patient reported outcome measures (PROMs), PoC and home-based tests to measure fecal calprotectin (FC) near the patient improved the ability to evaluate more types of patients with IBD at a distance, even in more complex cases.
The increase in the capacity of data transmission and storage, as well as the evolution of wireless communications provided many resources that are easy to use and adaptable to IBD telemonitoring.
Initially, telemonitoring systems for IBD allowed communication between health centres and patient´s home using computers. Afterwards, the development of web-based systems permitted easy-to-use and cheaper telemonitoring programs. In the last years, mobile devices (Smartphone, Tablet, etc.) made it possible to establish the communication process with the patient during his/her daily activities. Furthermore, in other settings (such as cardiovascular diseases) the transmission of continuous physiological data through biotelemetry has evolved with the incorporation of wearables.
In the IBD setting, telemonitoring is a safe, acceptable, and effective option to improve clinical outcomes[38,39], but the results of studies are still variable. In this context, telemonitoring has mainly used programs requiring home installation or web-systems, although e-mail and telephone have supported some of these programs. The main telemonitoring platforms used in IBD are summarized in Table 1.
| Ref. | Disease | Type of study | n | Application | Outcomes |
| Cross et al[21] | IBD | Noncontrolled, clinical trial | 10 | Telemonitoring, home unit-server PC provider | Feasible method |
| Excellent patient acceptance | |||||
| Cross et al[113] | IBD | Noncontrolled clinical trial | 25 | Telemonitoring, home unit-server PC provider | Feasible method |
| Excellent patient acceptance | |||||
| Improvement in QoL, disease activity, and disease knowledge | |||||
| Cross et al[42] | UC | Controlled randomized clinical trial | 47 | Telemonitoring, home unit-server PC provider | Feasible method |
| Excellent patient acceptance | |||||
| Improvement in QoL | |||||
| Elkjaer et al[115] | UC | Validation study in 2 groups | 21 | Telemonitoring through the web | Feasible method |
| Excellent patient acceptance | |||||
| Elkjaer et al[22] | UC | Controlled randomized clinical trial | 333 | Telemonitoring through the web | Feasible method |
| Excellent patient acceptance | |||||
| Improvement in QoL, disease knowledge, and adherence | |||||
| Pedersen et al[10] | CD | Pilot study, controlled | 27 | Telemonitoring through the web | Feasible and safe method for individualized scheduling of maintenance IFX treatment |
| Pedersen et al[11] | UC | Prospective noncontrolled study | 95 | Telemonitoring through the web | Feasible and improve adherence to therapy |
| Torrejón et al[56] | IBD | Descriptive, observational, retrospective | 1784 | Telecare through e-mail, phone calls, fax | Increased telematic contacts and decreased in-person care |
| Johnson et al[27] | IBD | Telemonitoring project | 420 | A web-guided programme | Effective, safe and cost savings |
| De Jong et al[24] | IBD | Controlled randomized clinical trial | 909 | Telemonitoring through the web (mHealth) | Reduced outpatient visits and hospitalizations |
| Carlsen et al[43] | IBD | Controlled randomized clinical trial | 53 | Telemonitoring through the web (mHealth) | Reduced outpatient visits |
| No differences in disease activity, QoL or adherence compared with standard care | |||||
| Walsh et al[112] | UC | Pilot study, non controlled | 66 | Telemonitoring through the web (mHealth) | Feasible and usable to measure disease activity, QoL and medication use |
| Del Hoyo et al[52] | IBD | Controlled randomized clinical trial | 63 | Telemonitoring through the web | Higher improvement in disease activity compared to usual care |
| Similar improvement in QoL, social activities and satisfaction between groups | |||||
| Cross et al[25] | IBD | Controlled randomized clinical trial | 348 | Telemonitoring through the web (mHealth) | Improvement in disease activity and QoL, although not superior to usual care |
| Decrease in hospitalizations and increase in distance contacts | |||||
| Bilgrami et al[48] | IBD | Controlled randomized clinical trial | 222 | Telemonitoring through the web (mHealth) | No differences in self-efficacy or patient activation compared with standard care |
| Schliep et al[47] | IBD | Controlled randomized clinical trial | 217 | Telemonitoring through the web (mHealth) | No significant improvement in depressive symptoms or QoL compared with standard care |
| Heida et al[45] | IBD | Controlled randomized clinical trial | 170 | Telemonitoring through the web, e-mail and telephone | Similar improvement in QoL compared to conventional care |
| Reduction in outpatient visits and societal costs | |||||
| Satisfaction | |||||
| Linn et al[46] | IBD | Controlled randomized clinical trial | 160 | Telemonitoring through the web or SMS combined with tailored counselling | Improved self-efficacy |
| Satisfaction | |||||
| Bonnaud et al[51] | IBD | Controlled randomized clinical trial | 54 | Telemonitoring through the web (mHealth) | Significant improvement in QoL |
| A trend to reduce outpatient visits | |||||
| Satisfaction | |||||
| McCombie et al[50] | IBD | Controlled randomized clinical trial | 100 | Telemonitoring through the web (mHealth) and home-based FC | Non-inferiority of QoL and symptoms |
| Reduced outpatient visits |
The Cross group was the first to apply ICTs in adult patients with IBD, mainly with UC. This research team developed a remote-control system (home automated telemanagement system: HAT system) made up of 3 stations, adapted from a program previously used in self-management of patients with asthma[40]. The Home Unit was made up of a portable computer that collected patients' information (symptoms, adverse effects, medication, etc.), and these data were then sent to a decision-support server connected to a provider’s PC[21]. The computer created alerts if the values collected in a web portal surpassed pre-established thresholds. Moreover, the HAT system incorporated educative elements.
Different exploratory studies showed good acceptance with the use of this system. In 2 studies with 10 and 23 patients with IBD, all of them considered that the HAT system was simple and increased patients´ knowledge[21,41]. To confirm the acceptability and adherence to follow-up with this program, the authors performed a subsequent study with 25 patients followed-up over 6 mo. Adherence to the weekly questionnaire was 91% and 86% had a prescribed medication adherence over 80%. This good adherence corresponded to a trend towards improvement in disease activity and QoL levels, together with a statistically significant improvement in understanding the disease (P = 0.0015). These good results led to the hypothesis that the HAT system could be feasible for telemonitoring patients with IBD.
Subsequently, the same group designed a randomized clinical trial including 47 patients with mild to moderate UC. Twenty-five patients were controlled with the HAT system and 22 followed usual in-person visits together with educational support and individualized action plans to make groups more comparable[42]. The groups had similar baseline characteristics, except for the use of immunosuppressants in 56% of the study group and 27% of the control group (P = 0.05), which would indicate a higher level of disease activity in the experimental group. There were no statistically significant differences for improvement of disease activity, treatment adherence, and quality-of-life values between both groups at 12 mo. These results could be related to the small sample size as well as a higher dropout rate in the intervention group, possibly due to the platform design, which required installation and eventual repairs at home.
To avoid these problems, Cross and cols developed web telemonitoring using mobile devices[25].
In the last decade, telemonitoring systems have progressively evolved with web programs and mHealth solutions. Web applications are less expensive, safe, and feasible in the management of IBD not only in adults but also in adolescents[43-45], and they are associated with a reduction in outpatient visits and hospitalizations[22,24,27,43,45].
A Danish group developed telemonitoring through the web under the concept of “Constant-care”. The system was developed through the web http://www.constant-care.dk, which also incorporated an educational centre. These investigators designed a randomized controlled trial with 333 UC patients treated with 5-aminosalicylates (5-ASA) from different hospitals in Denmark and Ireland[22]. The intervention group introduced clinical data and analytic results in the web to guide changes in the follow-up schedule and treatment. This intervention was compared to usual care.
After 12 mo of follow-up, in both the Danish and Irish population 88% of patients showed good acceptance with the web telemonitoring. There was a statistically significant improvement in adherence to treatment after 4 wk and a lower duration of disease flares. This was related to the use of high doses of 5-ASA in 100% of patients from the intervention group in Denmark, who also had improved QoL and disease knowledge. However, these outcomes were not reproduced in the Irish population. Moreover, in the Danish population telemonitoring reduced outpatient and emergency department visits, which led to direct cost-savings of 189 euros per patient-year, but also to an increase of e-mails and telephone contacts.
The use of this web-system in paediatric patients also reduced outpatient visits and school absenteeism, without differences in disease activity, QoL and adherence to treatment compared to the control group[43]. In another study developed in the University of California with a telemonitoring program combined with tele-education, patients followed remotely used less corticosteroids and suffered less hospitalizations and emergency department visits, with cost reductions of 16%[23]. In short, these studies show that web-telemonitoring is feasible, safe and could reduce health costs, although there are reproducibility differences depending on the population in which telemonitoring is applied[19].
Moreover, web-systems have been used to individualize the treatment according to the disease course. In a prospective study with 95 patients with mild to moderate UC, web control allowed the adjustment of 5-ASA doses and improved adherence. This was related with a significant improvement in clinical activity and FC values after 3 mo of follow-up[11]. Telemonitoring has even been used to individualize the treatment schedule with infliximab. After 1 year of follow-up, there were no significant changes in disease activity and QoL, although there was an estimated cost-saving of 699 euros/patient, compared with a historic control group[10].
In the same line and to avoid problems generated with the HAT system in the pioneering studies, the Cross group developed a web system for the management of patients with IBD (TELE-IBD) through text messages. In a randomized clinical trial with 3 parallel groups (TELE-IBD weekly, TELE-IBD every other week and control group) in 3 reference centres for IBD, they included 348 patients who had at least one disease flare in the last 2 years. Seventy-five percent completed the study, with an improvement in disease activity and QoL in the 3 groups, but without a higher improvement in these outcomes, depressive symptoms, or self-efficacy in the web control group, although in another study self-efficacy improved when tailored counselling was associated[46]. Moreover, telemonitoring was associated with a change in the profile of health expenses. Less hospitalizations were seen in the telemonitoring group but with higher use of non-invasive tests and telephone or e-mail[25,47,48].
The largest clinical trial with a telemonitoring program to date was performed with the Dutch web myIBDcoach (http://www.mijnibdcoach.nl). This web allows distance monitoring of disease activity, treatment adherence and side effects, as well as nutritional status, smoking habits, QoL, fatigue, stress, anxiety and depression. As other platforms, it provides educational elements to improve empowerment. Patients showed good acceptance with its use in a pilot study[49]. In a clinical trial including 909 patients with different disease characteristics, the use of this system reduced outpatient visits and hospitalizations compared to standard care after 12 mo of follow-up[24]. Similarly, a reduction in outpatient visits was also obtained in adolescents[43,45] and in adults who used home-based tests to measure FC[50]. In a pilot study performed in France with the EasyMICI-MaMICI® platform, a reduction in outpatient visits was also associated with a significant improvement in QoL and satisfaction[51].
Our study group evaluated the impact on health outcomes of the telemonitoring web platform TECCU (Telemonitorización de la Enfermedad de Crohn y Colitis Ulcerosa or Telemonitoring of Crohn’s Disease and Ulcerative Colitis), as compared to standard care and telephone care. In a 3-arm randomized clinical trial, 63 patients (21 per arm) with complex IBD were managed with each follow-up method over 24 wk. At the end of the study, the percentage of patients in remission was higher in the TECCU group (17/21, 81%) compared to nurse assisted telephone care (14/21, 66.7%) and standard care (15/21, 71.4%). The telemonitoring group had more improvement in disease activity, and this was associated with a larger reduction in FC values. All completers adhered to treatment in the TECCU group, while QoL, social activities, and satisfaction improved in all 3 groups[52].
Telephone and e-mail are resources attended by both medical doctors and specialized nurses in some IBD units, with high capacity to solve problems at less cost[53-55]. These tools have also been used to coordinate action plans in telemonitoring systems.
In Spain, the Crohn´s and Colitis Care Unit model has been used since 1999 as a multidisciplinary model of continuous care for patients with IBD. This model manages health demands with distance management mainly through telephone or e-mail with the support of a web page, which includes educational elements. The number of users has risen over the years, with a reduction of in-person care[56]. In Illinois, the Sonar Project is based on monthly web monitoring of symptoms in patients with IBD. Nurses exert a central role and use telephone contact for those patients who send results out of normal ranges, and together with medical health providers management adjustments are performed. This system also reduced hospitalizations, emergency visits and costs[57].
Therefore, beyond the use of telephone and e-mail in units which work as centres for resource coordination, telemedicine in IBD is expanding through the use of web and mHealth systems. These include telemonitoring, tele-education and videocalls in some cases. Its application allows the development of projects to provide health resources in remote areas[58,59], mainly with the use of mobile apps and the integration of some of these platforms into the electronic medical records, as is the case of the app HealthPROMISE and mynexuzhealth[60,61]. These models promote collaboration and mentoring between specialists, which could reduce variability in medical practice and modify the structure of future health systems if they demonstrate to be cost-effective.
Although many data about cost-savings have been published, they refer almost exclusively to direct costs[22,27], without considering costs of installation and maintenance of platforms or indirect costs.
In the IBD setting, our research group published the first cost-effectiveness and cost-utility analysis of a telemonitoring program compared to telephone and standard care[62,63]. The differences between groups and statistical uncertainty in disease activity, quality-adjusted life-years, and costs were calculated using nonparametric bootstrap estimations. Even though our trial only included 63 patients, we imputed the original dataset 5 times, and the bootstrapping estimations allowed us to extract 1000 random samples (of 21 patients per arm) from each of the 5 imputations, thus generating 5000 bootstrap replications.
We concluded that there is a high probability (79.96%) that the use of the TECCU web platform for telemonitoring complex IBD patients produces a greater improvement in disease activity at a lower societal cost, compared with standard care. Telemonitoring through the TECCU platform saved €2250 per additional patient in remission (95%CI: €–15363 to 11086) vs telephone care, and telephone care saved €538 compared with standard care (95%CI: €–6475 to 5303). Moreover, the use of the TECCU platform and telephone care showed an 84% and 67% probability, respectively, of producing a cost saving per additional quality-adjusted life-year (QALY) compared with usual care, even considering the simulations that involved negative incremental QALYs.
With a similar methodology, our results were reproduced by de Jong et al[64] who concluded that telemedicine with myIBDcoach is cost-saving and has a high probability of being cost-effective, without a decline in QoL. In this study, telemedicine resulted in lower mean annual costs of €547/patient (95%CI: €1029-2143) without changing quality adjusted life years. At the Dutch threshold of €80000 per quality adjusted life year, the intervention had an increased incremental cost-effectiveness over standard care in 83% of replications.
The authors included all subtypes of IBD, whereas our study with the TECCU platform recruited complex patients with IBD who needed to start immunosuppressants and/or biologic agents. According to our conclusions, the big sample size recruited in this article is useful to confirm our prior results, and the reproducibility of the favourable cost-effectiveness profile of telemedicine applied in IBD across countries and patients’ characteristics. In fact, during the COVID-19 pandemic, similar cost-savings with a higher gain of QALYs have been observed with the use of telemonitoring for IBD in Hong Kong[65].
Unlike the use of ICTs in other fields (streaming entertainment services, grocery delivery, e-banking,
| Enablers | Barriers | |
| Technological | Adequate support | Lack of EMR integration |
| Sufficient training | System maintenance required to avoid malfunction | |
| Fast internet connections | ||
| 5G network | ||
| Organizational | Continuous monitoring | Multidimensional nature: complex comparability between programmes |
| Overcome geographic barriers | ||
| Safe assistance during COVID-19 pandemic | Lack of robust data: small studies, short-term follow up periods | |
| Structured data collection | ||
| Favours experimental studies and epidemiological surveillance | Lack of standardized remote medical practice (Interstate Mecial Licensure compact in the United States)[66] | |
| Multicentric access to data | ||
| Reimbursement limitations | ||
| Telementoring: professional support and education | ||
| Legal | Lack of legal framework[67,68] | |
| Data security | ||
| Acceptability/accessibility | Patient empowerment | Technological knowledge[69-71]; Some demographic factors increase the likelihood of a telematic encounter failure |
| High drop-out rate in some clinical trials | ||
| Wide use of smartphones | ||
| Wide use of wearable devices | ||
| Cheap internet plans | ||
| Costs | Potential decrease of direct and indirect costs | High initial investment |
| Limited cost-effectiveness data |
Telemonitoring theoretically include three different diagnostic models: patient self-diagnosis, remote providers´ diagnosis and computer-assisted diagnosis. They usually work as triage systems but, beyond diagnostic capabilities, telemonitoring platforms allow for remote management of other aspects such as disease treatment or education. In the IBD setting, they usually combine self-management, remote providers´ management and computer-assisted telemanagement[10,11,21,22,24,25,42,43,45,47,48,50-52].
Self-management refers to a dynamic, interactive, and daily process in which individuals engage to manage a chronic illness[73]. This process includes the ability to deal with their own symptoms, treatment, physical and social consequences, and lifestyle changes to maintain a satisfactory QoL[74]. In this sense, telemonitoring platforms for IBD have incorporated PROMS and home-based tests that allowed patients to self-report their health status. This information has been even used to guide treatment adjustments by themselves[22].
Considering the “treat-to-target” strategy, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) project recognized different evidence and consensus-based recommendations to optimize outcomes in patients with IBD. Among the different targets proposed, clinical remission and endoscopic healing were confirmed in the STRIDE-II actualization, while normalization of serum and fecal markers of inflammation have been determined as short-term targets[4]. There was agreement to evaluate disease remission with clinical indexes, PROMs and endoscopic criteria [or also radiologic criteria in Crohn's disease (CD)].
Usually, endoscopic disease activity has been considered the gold standard to measure inflammation and to consider mucosal healing, but endoscopy is invasive and expensive.
In this sense, with the aim of measuring inflammation non-invasively, different PROMs and PoC tests have been developed over last years. Moreover, some of these tools have been specifically validated for their use in telemedicine programs.
PROMs: A PROM is a measurement of any aspect of a patient's health status that comes directly from the patient, without the interpretation of the patient's responses by healthcare providers and without the need of laboratory tests[75]. PROMs are designed for screening of disease activity, and then they need to be sensitive enough, especially if it implies more false positive results.
The Simple Clinical Colitis Activity Index (SCCAI) has shown a high correlation and good agreement between patient and clinician reported versions[76]. Compared to UC, PROMs used in the context of CD have shown worse correlation with other markers of clinical or endoscopic activity. The Harvey-Bradshaw index (HBI) had high correlation but only moderate agreement between versions registered by the patient or the clinician[77], although a recent version of the HBI self-administered by the patient through a mobile app showed a high percentage of agreement with in-clinic physician assessment, with a remarkably high PPV for remission[78]. Both SCCAI and HBI show good agreement between paper and online versions[78-80], and represent attractive tools for telemonitoring IBD.
Few PROMs have had their correlation with endoscopic activity evaluated. The global assessment of the patient, based on an analogic visual scale about how they felt regarding their UC during the previous 2 d, only showed moderate correlation with endoscopic activity[81]. The subscore of the global medical assessment and the 6-point Mayo index (which includes stool frequency and rectal bleeding) have a high correlation with the whole Mayo index[82]. Moreover, the 6-point Mayo index has an AUC of 0.80 compared to the endoscopic subscore[83].
Recently, the mobile Health Index was validated to monitor IBD activity through mHealth systems. In patients with CD, it showed high correlation and agreement with the Crohn´s Disease Activity Index and the HBI, as well as in patients with UC when it was compared to the partial Mayo index[84]. The intraclass correlation coefficient for test-retest reliability was high for CD and for UC. Nevertheless, its agreement with endoscopic scores was poor in CD and moderate in UC.
QoL and absence of disability are other targets of the STRIDE-II initiative. The Inflammatory Bowel Disease Questionnaire (IBDQ) was specifically validated in patients with IBD and has a moderate to high correlation with treatment response and the endoscopic Mayo index. However, their 32 and 36 items versions require a lot of time for their interpretation, so the reduced versions of 9 and 10 questions were subsequently validated[85,86]. On the other hand, the IBD disability index predicts active disease, nonadherence, and treatment with corticosteroids when high disability values are obtained[87]. Finally, health-related fatigue was incorporated in the Monitor IBD At Home index, but it is still not considered a specific target.
Probably the accuracy of PROMs increases when used in combination with FC. Thus, the Monitor IBD At Home index was developed to predict the endoscopic activity in patients with IBD. The association of FC to both the CD and UC versions showed high sensibility and NPV to rule out endoscopic activity[88]. The development of home-based FC tests that can be measured by the patient represent a potential option to measure disease activity in telemonitoring programs.
PoC tests and home-based tests: The use of PoC tests refers to patient specimens assayed at or near the patient with the assumption that test results will be available instantly or in a very short timeframe to assist caregivers with immediate diagnosis and/or clinical intervention[89]. In the IBD setting, the interest has centered on FC, and even though lactoferrin tests have been developed with adequate accuracy[90], FC offers better sensibility at certain cutoffs[90,91].
FC has good correlation with endoscopic activity in both UC[92,93] and CD[94-96]. FC helps to differentiate between functional and inflammatory diseases in patients with digestive symptoms. Moreover, in patients already diagnosed of IBD it allows the evaluation of disease activity, response to treatment, post-surgical recurrence and it predicts relapses after the withdrawal of anti-TNF agents[97,98]. These features, its non-invasiveness and a relative low cost makes FC tests in a useful tool in the diagnosis, monitoring and treatment adjustment in IBD.
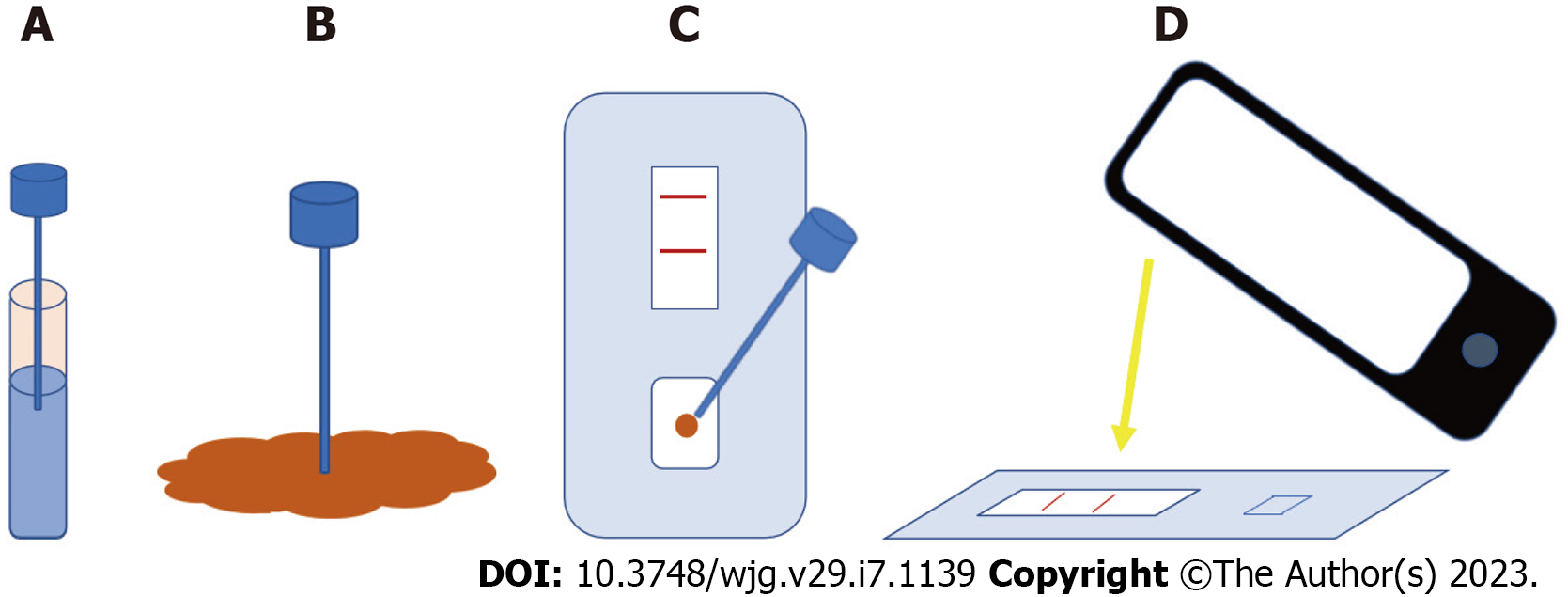
Furthermore, the diagnostic accuracy of FC in different clinical scenarios has increased the interest in its use in telemonitoring IBD programs. In line with a patient-centered care and to favour empowerment, during the last years and the COVID-19 pandemic, different home-based FC tests have been developed as an additional tool for a home-based follow-up[99]. These tests are based on kits that analysed faecal samples through immunochromatography. Then, the results are read with a smartphone camera, and they are sent through a specific app to a server accessible by providers (Figure 2).
Comparison between different home-based FC tests: Three main FC tests have been developed for its use by patients at home: CalproSmart, IBDoc and QuantonCal.
In a recent study, these 3 tests were compared with the ELISA method of the same manufacturer[100]. Considering the importance of obtaining good agreement in the low range of FC values (ruling-out disease activity), IBDoc, QuantonCal and CalproSmart have an 87%, 82% and 76% agreement, respectively, compared with their corresponding ELISA readings.
However, and similarly to its validation study[101], CalproSmart showed a trend to overestimate FC values with a mean bias of +141 µg/g (95%CI: -316 to 598 µg/g), while IBDoc tended to underestimate FC values with a mean bias of -105 µg/g (95%CI: -576 to 366 µg/g). This could generate more false positive results when using CalproSmart, but misclassification in the low (< 250 µg/g), medium (250-500 µg/g) or high (> 500 µg/g) range of FC values with this test showed large differences (i.e., to classify as > 500 with CalproSmart and < 250 with ELISA, and viceversa) in only 2% of measures, compared to 5% in IBDoc and 8% in QuantonCal.
In any case, the error range between FC measured with home-based tests and their corresponding ELISA method was high. This happens especially when FC values are > 500 µg/g. With values ≤ 500 µg/g, differences were also over the acceptable range of 200 µg/g (+/-100 µg/g), but they were not wide enough to induce errors in the interpretation of inflammatory activity. Therefore, home-based tests are considered useful to rule-out inflammation at a distance when FC values are < 500 µg/g, but when values are > 500 µg/g disease activity should be evaluated with other methods.
Home-drug monitoring: Home therapeutic drug monitoring of monoclonal antibodies appears to be an innovative possibility to improve and simplify IBD management. To date, these tests are performed only in some hospital laboratories and results are not immediate. This delay, of months in some cases, impairs drug monitoring and dose adjustment, compromising its utility.
To solve this issue, home drug monitoring using dried blood samples is being evaluated in different inflammatory diseases. The first data were published by Kneepkens et al[102] in patients with rheumatic inflammatory diseases. Adalimumab and anti-adalimumab antibodies concentration measurements in finger prick dried blood spots were compared with simultaneous serum measurements. They found that both drug levels and antibody concentrations from the finger prick method correlated well with serum measurements (correlation coefficient > 0.87). However, some disadvantages should be considered, such as loss in precision, workload and elevated costs.
Berends and colleagues did a similar study with 40 IBD patients, comparing infliximab concentrations in dried blood samples and serum. Home-based test infliximab concentrations showed a good correlation (correlation coefficient: 0.671) with serum measurements[103]. This author also published data with adalimumab treatment one year later. A high correlation was found (Pearson’s correlation: ≥ 0.96) between dried blood test and venipuncture results when performed at the same time during the outpatient clinic. Moderate correlation (Pearson coefficient = 0.51) was reported between home self-performed finger test and estimated adalimumab concentrations[104]. Larger studies are needed to confirm the reliability, accuracy, and cost effectiveness of home-based testing as a new telemonitoring tool in IBD.
Wearable devices: Wearable devices are electronic gadgets that consumers wear to track health-relevant physiological data to monitor and improve health[105]. IBD patient preferences and interest in wearable technology were evaluated by Hirten and colleagues using a 28-question survey. Four hundred patients completed the survey. Of these, 42.7% reported prior or current use of wearable devices, mainly smart watches (34.5%) and wrist band devices (29.1%). Almost 90% of subjects believed these gadgets could provide important information about their health and 93.8% reported that they would use them if it could help doctors manage their disease[106].
In the last few years, several studies have tried to demonstrate the utility of wearable devices in IBD telemonitoring[107]. The first available data was published by Yvellez et al[108] in 2018. They prospectively assessed daily health-related QoL, pain and sleep data using validated indexes through a mobile application and a Fitbit® device. Fitbit® compliance was almost 80%, suggesting this technology is feasible[108]. The Fitbit® device has also been used to predict disease activity. In one study a significant reduction in daily steps has been shown over the week before CRP or FC elevation, but without differences in daily resting heart rate[109]. Two years later, however, Hirten et al[110] demonstrated that significant changes in heart rate variability measured using VitalPatch® were observed before the development of symptomatic or inflammatory flare in ulcerative colitis patients.
Step count and sleep monitoring have also been used, not to predict flares but the intent to determine post-operative length of stay. Overall, step count and sleep duration/efficiency did not predict length of stay. However, in a multivariable linear regression model, significant interaction was found between postoperative complications and step count, suggesting that increased physical activity was associated with a reduction in duration of hospital stay[111].
All the telemonitoring interventions in IBD reported in this review comprise healthcare providers´ advice. Most systems employ store and forward programs, where nurses acquire a central role in tracking the information received and making contact between patients and specialists to set up healthcare plans. Communication is established through websites, usually with the support of telephone and e-mail, as reported above.
Computerized systems have been used for telemonitoring IBD and they usually work as a triage system to identify which patients might require further evaluation. Many of them can generate automatic action plans through the integration of different monitorable indicators in decision-making algorithms. In most telemonitoring programs tested thus far, these tools are combined with self-management and the remote providers´ management models seen above.
Telemonitoring systems in IBD are integrated by personal computers or mobile devices used by patients, a decision support server and a website for staff and providers. The website provides an interface to collect data from testing sessions, and these platforms usually generate automated reminders to favour adherence to follow-up. The structure of most eHealth tools in IBD are based on a traffic light system[10,11,22,25,27,43,45,52,112]. Patients usually enter their symptoms in scheduled online controls, mainly in a structured manner through PROMS, but many apps also include a comment box to freely express anything outside the questionnaires. Then, the patient´s status appears as red, yellow or green when disease is highly, moderately active, or quiescent, respectively.
Some systems combine self-reported symptoms with the level of FC in a total inflammation burden score. In fact, recent clinical trials have incorporated FC tests performed by patients at home[45,50,112]. This status is sometimes supplemented with disease activity and QoL graphs[10,11,22]. Depending on the level of alert, simultaneous action plans and email alerts are sent to the participant and providers, who review the information to decide if further management changes are necessary.
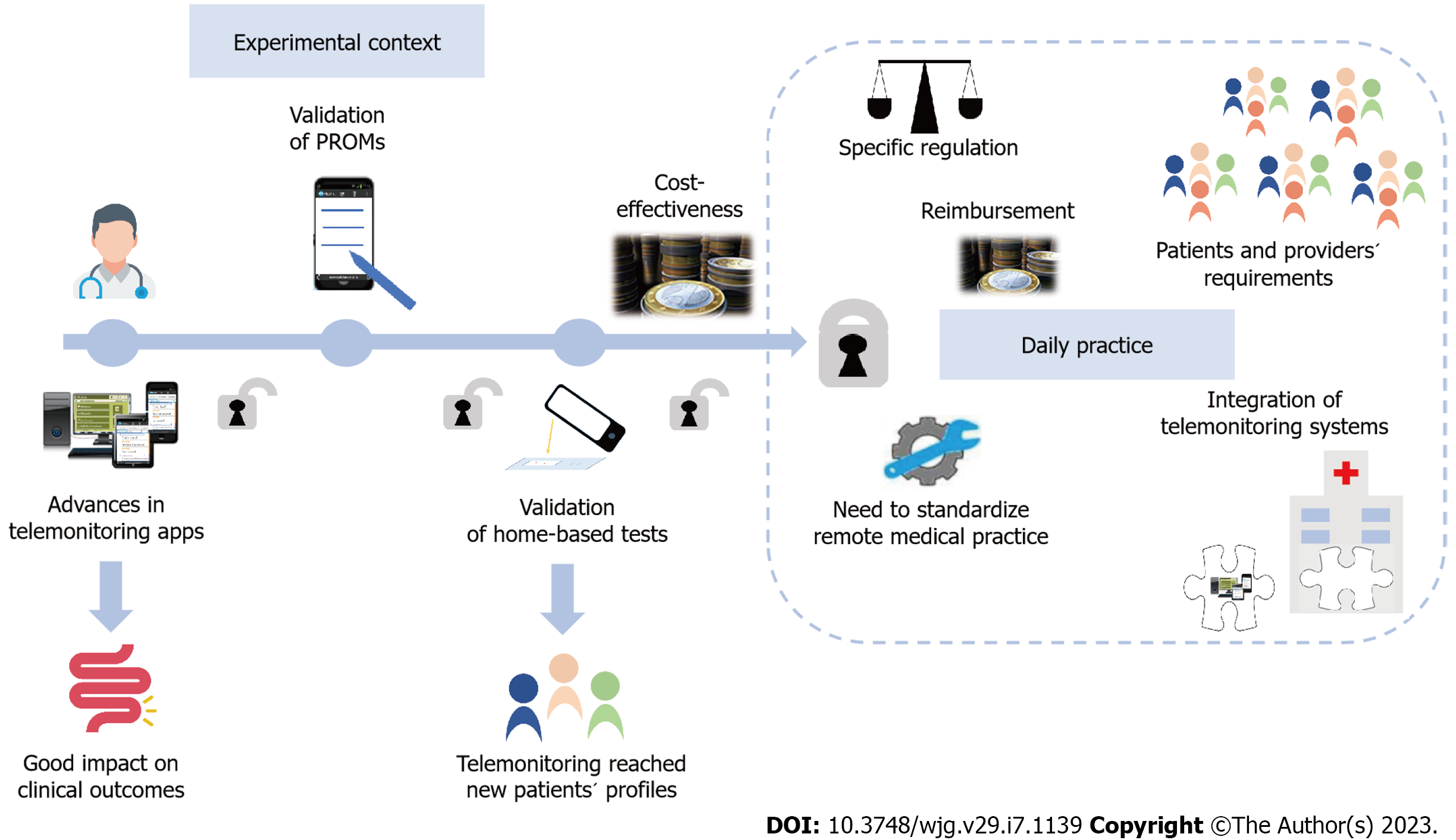
Altogether, these new models and resources for patients´ self-management represent advances to reach the implementation of telemonitoring in daily practice, but still some legal, ethical, economical and logistic barriers need to be solved (Figure 3).
Telemonitoring is about communication, and the development of faster and wireless systems at a lower cost has supported the use of proactive remote monitoring. As well, the increase in data storage also favoured the incorporation of tele-education in most telemonitoring programs in the IBD setting. However, during the pandemic, e-mail and telephone still represented the main resources used[29,30], while the application of mature telemonitoring programs was the exception. As different enablers encouraged advances of telemonitoring in IBD, many other barriers emerged and hindered its full implementation in daily practice.
Pioneering studies evaluated telemonitoring programs previously used in other chronic diseases[21,42,113,114]. Feasibility and patients´ acceptance of these applications was excellent. Yet, they were not able to clearly demonstrate an improvement in QoL, disease activity, and treatment adherence. These systems were based on remote monitoring through computers, they needed to be adapted to the IBD context and required eventual repairs at home, which were time expensive. Telemonitoring subsequently evolved towards the use of web-based systems, which were cheaper and easy to use. Remote monitoring through the web demonstrated its feasibility and excellent patients’ acceptance, with an improvement in QoL, disease activity, and disease knowledge[22,115].
In the last few years, telemonitoring prioritized the use of mHealth resources[24,25,116]. Beyond the improvement in clinical outcomes, mHealth telemonitoring associated cost-savings in outpatient visits and hospitalizations[23-26]. Almost parallel to the mHealth evolution, many PROMs have been validated to self-report disease activity. In line with patient-centered care, empowered patients can use new home-based calprotectin tests, which are accurate enough and useful to rule-out disease activity at low FC values[100,101]. In fact, new home drug monitoring is being developed to measure levels of monoclonal antibodies near the patient[103,104]. Furthermore, the development of mobile devices even enabled the increasing use of wearables to monitor physiological predictors of disease activity and postoperative length of stay[110,111]. These new tools could represent one of the first steps towards ubiquitous Health in IBD, and in a near future machine learning may allow the integration of large data sets in personalized algorithms.
Despite the technological and knowledge advances reached, the effect of telemonitoring on health outcomes is not consistent in different populations and health systems[22,24,25,42,44,45,52]. Initially, remote monitoring was mostly restricted to UC patients, while the design of new apps, PROMs and home-based tests allowed to progressively expand its use to a broad range of patients´ profiles. However, telemonitoring has not been demonstrated to improve QoL or clinical/endoscopic remission in the long-term. In addition, biomarkers in IBD are less accurate compared to other chronic diseases such as diabetes mellitus, and the early recognition of complications in IBD still require invasive tests in many cases.
On the other hand, although telemedicine has been traditionally considered cost-effective, cost-saving data previously published referred almost exclusively to direct costs[22,27]. The implementation of telemonitoring services represents a short-term high initial cost, not only from a technological point of view, but also by changes in the organization of the IBD units. Thus, decision-makers have had difficulties to support the implementation and investment in telemedicine due to a lack of solid evidence so far. In addition, these decisions become even more complicated in areas where rei
The availability of more powerful and cheaper communication tools turned technical challenges into legal, ethical, economical, and logistic issues[28]. To standardize remote medical practice, in the US the Interstate Medical Licensure Compact was created to increase efficiency in multistate licensing of physicians[66] but such a proposal is lacking in Europe. Besides, only a few examples of full integration of telemonitoring programs into electronic medical records are available to date[60,61]. In this sense, interoperability of systems while maintaining the confidentiality of data cannot be guaranteed in many centres. Moreover, the provision of remote health safely also requires a specific European regulation to protect remote medical practice and to lift some existing legal barriers. Finally, to keep adherence to follow-up, it is essential to adapt telemedicine programs according to patients’ and providers´ characteristics, because some demographic factors such as increasing age, commercial insurance status and racial differences increase the likelihood of a telematic encounter failure in some contexts[117].
Therefore, telemonitoring IBD is well accepted and improves clinical outcomes at a lower cost in the short-term. The advances performed on new PROMS, home-based tests and wearables improved the ability to manage new patients´ profiles remotely. However, it is still necessary to overcome many legal, ethical, economical and logistic barriers. Funders, policymakers, providers and patients need to align their interests to successfully implement telemonitoring, and further collaborative efforts based on teamwork between centres are essential to help reorganize health systems.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao CH, China; Dai YC, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T; IBSEN Group. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. 2013;62:368-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Hoivik ML, Moum B, Solberg IC, Cvancarova M, Hoie O, Vatn MH, Bernklev T; IBSEN Study Group. Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis. 2012;18:1540-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2677] [Cited by in F6Publishing: 3220] [Article Influence: 460.0] [Reference Citation Analysis (0)] |
| 4. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 936] [Article Influence: 312.0] [Reference Citation Analysis (0)] |
| 5. | Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105:525-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents' perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:589-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | van Deen WK, van Oijen MG, Myers KD, Centeno A, Howard W, Choi JM, Roth BE, McLaughlin EM, Hollander D, Wong-Swanson B, Sack J, Ong MK, Ha CY, Esrailian E, Hommes DW. A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1747-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kappelman MD, Porter CQ, Galanko JA, Rifas-Shiman SL, Ollendorf DA, Sandler RS, Finkelstein JA. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Pedersen N, Elkjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S, Knudsen T, Thorsgaard N, Munkholm P. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:840-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Pedersen N, Thielsen P, Martinsen L, Bennedsen M, Haaber A, Langholz E, Végh Z, Duricova D, Jess T, Bell S, Burisch J, Munkholm P. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20:2276-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Kitsiou S, Paré G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: An overview of systematic reviews. J Med Internet Res. 2015;17:e63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Jayakody A, Bryant J, Carey M, Hobden B, Dodd N, Sanson-Fisher R. Effectiveness of interventions utilising telephone follow up in reducing hospital readmission within 30 days for individuals with chronic disease: a systematic review. BMC Health Serv Res. 2016;16:403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth Interventions to Support Self-Management of Long-Term Conditions: A Systematic Metareview of Diabetes, Heart Failure, Asthma, Chronic Obstructive Pulmonary Disease, and Cancer. J Med Internet Res. 2017;19:e172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 15. | Greenwood DA, Young HM, Quinn CC. Telehealth Remote Monitoring Systematic Review: Structured Self-monitoring of Blood Glucose and Impact on A1C. J Diabetes Sci Technol. 2014;8:378-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;CD007718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Lundell S, Holmner Å, Rehn B, Nyberg A, Wadell K. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med. 2015;109:11-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | McLean S, Chandler D, Nurmatov U, Liu J, Pagliari C, Car J, Sheikh A. Telehealthcare for asthma. Cochrane Database Syst Rev. 2010;CD007717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Aguas Peris M, Del Hoyo J, Bebia P, Faubel R, Barrios A, Bastida G, Valdivieso B, Nos P. Telemedicine in inflammatory bowel disease: opportunities and approaches. Inflamm Bowel Dis. 2015;21:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Helsel BC, Williams JE, Lawson K, Liang J, Markowitz J. Telemedicine and Mobile Health Technology Are Effective in the Management of Digestive Diseases: A Systematic Review. Dig Dis Sci. 2018;63:1392-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Cross RK, Arora M, Finkelstein J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006;40:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, Avnstrøm S, Langholz E, O'Morain C, Lynge E, Munkholm P. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided 'Constant-care' approach. Gut. 2010;59:1652-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | van Deen WK, Spiro A, Burak Ozbay A, Skup M, Centeno A, Duran NE, Lacey PN, Jatulis D, Esrailian E, van Oijen MG, Hommes DW. The impact of value-based healthcare for inflammatory bowel diseases on healthcare utilization: a pilot study. Eur J Gastroenterol Hepatol. 2017;29:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, Becx MC, Maljaars JP, Cilissen M, van Bodegraven AA, Mahmmod N, Markus T, Hameeteman WM, Dijkstra G, Masclee AA, Boonen A, Winkens B, van Tubergen A, Jonkers DM, Pierik MJ. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390:959-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 25. | Cross RK, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Collins JF, Katz J, Ghazi L, Patil SA, Quezada SM, Beaulieu D, Horst SN, Russman K, Riaz M, Jambaulikar G, Sivasailam B, Quinn CC. A Randomized Controlled Trial of TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD). Am J Gastroenterol. 2019;114:472-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Zhen J, Marshall JK, Nguyen GC, Atreja A, Narula N. Impact of Digital Health Monitoring in the Management of Inflammatory Bowel Disease. J Med Syst. 2021;45:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Johnson MW, Lithgo K, Price T. OC-080 Ibd-sshamp (supported, self help and management programme); UK’S first internet based remote management system for managing stable IBD. Gut. 2013;62 (Suppl 1):A34-A35. [DOI] [Cited in This Article: ] |
| 28. | Del Hoyo J, Aguas M. Implementing telemedicine in inflammatory bowel disease: Is COVID-19 the definitive trigger? Gastroenterol Hepatol. 2020;43:415-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Martin Arranz E, Suarez Ferrer C, García Ramírez L, Rueda García JL, Sánchez-Azofra M, Poza Cordón J, Noci J, Zabana Y, Barreiro-de Acosta M, Martín-Arranz MD. Management of COVID-19 Pandemic in Spanish Inflammatory Bowel Disease Units: Results From a National Survey. Inflamm Bowel Dis. 2020;26:1149-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Del Hoyo J, Millán M, Garrido-Marín A, Nos P, Barreiro-de Acosta M, Bujanda L, de la Portilla F, Aguas M; AEG, AECP, GETECCU. Changes in the management of IBD patients since the onset of COVID-19 pandemic. A path toward the implementation of telemedicine in Spain? Gastroenterol Hepatol. 2022;45:697-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Morton K, Dennison L, May C, Murray E, Little P, McManus RJ, Yardley L. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100:616-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 32. | Marcolino MS, Oliveira JAQ, D'Agostino M, Ribeiro AL, Alkmim MBM, Novillo-Ortiz D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth. 2018;6:e23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 512] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 33. | Peretz D, Arnaert A, Ponzoni NN. Determining the cost of implementing and operating a remote patient monitoring programme for the elderly with chronic conditions: A systematic review of economic evaluations. J Telemed Telecare. 2018;24:13-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Stefanopoulou E, Lewis D, Taylor M, Broscombe J, Ahmad J, Larkin J. Are Digitally Delivered Psychological Interventions for Depression the Way Forward? Psychiatr Q. 2018;89:779-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Linardon J, Fuller-Tyszkiewicz M. Attrition and adherence in smartphone-delivered interventions for mental health problems: A systematic and meta-analytic review. J Consult Clin Psychol. 2020;88:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 36. | McCombie A, Gearry R, Andrews J, Mulder R, Mikocka-Walus A. Does Computerized Cognitive Behavioral Therapy Help People with Inflammatory Bowel Disease? Inflamm Bowel Dis. 2016;22:171-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Bourbeau J, Echevarria C. Models of care across the continuum of exacerbations for patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2020;17:1479973119895457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Yin AL, Hachuel D, Pollak JP, Scherl EJ, Estrin D. Digital Health Apps in the Clinical Care of Inflammatory Bowel Disease: Scoping Review. J Med Internet Res. 2019;21:e14630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 39. | George LA, Cross RK. Remote Monitoring and Telemedicine in IBD: Are We There Yet? Curr Gastroenterol Rep. 2020;22:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Finkelstein J, O'Connor G, Friedmann RH. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Stud Health Technol Inform. 2001;84:810-814. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Castro HK, Cross RK, Finkelstein J. Using a Home Automated Telemanagement (HAT) system: experiences and perceptions of patients with inflammatory bowel disease. AMIA Annu Symp Proc. 2006;872. [PubMed] [Cited in This Article: ] |
| 42. | Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT). Inflamm Bowel Dis. 2012;18:1018-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Carlsen K, Jakobsen C, Houen G, Kallemose T, Paerregaard A, Riis LB, Munkholm P, Wewer V. Self-managed eHealth Disease Monitoring in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm Bowel Dis. 2017;23:357-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Carlsen K, Houen G, Jakobsen C, Kallemose T, Paerregaard A, Riis LB, Munkholm P, Wewer V. Individualized Infliximab Treatment Guided by Patient-managed eHealth in Children and Adolescents with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1473-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Heida A, Dijkstra A, Muller Kobold A, Rossen JW, Kindermann A, Kokke F, de Meij T, Norbruis O, Weersma RK, Wessels M, Hummel T, Escher J, van Wering H, Hendriks D, Mearin L, Groen H, Verkade HJ, van Rheenen PF. Efficacy of Home Telemonitoring versus Conventional Follow-up: A Randomized Controlled Trial among Teenagers with Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:432-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Linn AJ, van Dijk L, van Weert JCM, Gebeyehu BG, van Bodegraven AA, Smit EG. Creating a synergy effect: A cluster randomized controlled trial testing the effect of a tailored multimedia intervention on patient outcomes. Patient Educ Couns. 2018;101:1419-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Schliep M, Chudy-Onwugaje K, Abutaleb A, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Ghazi L, Patil SA, Quezada S, Russman K, Horst S, Beaulieu D, Quinn C, Jambaulikar G, Cross RK. TELEmedicine for Patients With Inflammatory Bowel Disease (TELE-IBD) Does Not Improve Depressive Symptoms or General Quality of Life Compared With Standard Care at Tertiary Referral Centers. Crohns Colitis 360. 2020;2:otaa002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Bilgrami Z, Abutaleb A, Chudy-Onwugaje K, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Ghazi L, Patil SA, Quezada SM, Russman KM, Quinn CC, Jambaulikar G, Beaulieu DB, Horst S, Cross RK Jr. Effect of TELEmedicine for Inflammatory Bowel Disease on Patient Activation and Self-Efficacy. Dig Dis Sci. 2020;65:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | de Jong M, van der Meulen-de Jong A, Romberg-Camps M, Degens J, Becx M, Markus T, Tomlow H, Cilissen M, Ipenburg N, Verwey M, Colautti-Duijsens L, Hameeteman W, Masclee A, Jonkers D, Pierik M. Development and Feasibility Study of a Telemedicine Tool for All Patients with IBD: MyIBDcoach. Inflamm Bowel Dis. 2017;23:485-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | McCombie A, Walmsley R, Barclay M, Ho C, Langlotz T, Regenbrecht H, Gray A, Visesio N, Inns S, Schultz M. A Noninferiority Randomized Clinical Trial of the Use of the Smartphone-Based Health Applications IBDsmart and IBDoc in the Care of Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. 2020;26:1098-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Bonnaud G, Haennig A, Altwegg R, Caron B, Boivineau L, Zallot C, Gilleta de Saint-Joseph C, Moreau J, Gonzalez F, Reynaud D, Faure P, Aygalenq P, Combis JM, Peyrin-Biroulet L. Real-life pilot study on the impact of the telemedicine platform EasyMICI-MaMICI® on quality of life and quality of care in patients with inflammatory bowel disease. Scand J Gastroenterol. 2021;56:530-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Del Hoyo J, Nos P, Faubel R, Muñoz D, Domínguez D, Bastida G, Valdivieso B, Correcher M, Aguas M. A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients With Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial. J Med Internet Res. 2018;20:e11602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Sanromán Alvarez L, de Castro Parga ML, Hernández Ramírez V, Pineda Mariño JR, Salgado Alvarez C, Rodríguez Grégori JM. [Telematic consultations by nursing staff for patients with inflammatory bowel disease: evaluation of its capacity for resolving problems and its costs]. Enferm Clin. 2014;24:102-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Bager P. The impact of nurse-led annual telephone follow-up of patients with inflammatory bowel disease. BMJ Qual Improv Rep. 2014;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Navarro Correal E, Benítez Leiva O, Dosal Galguera A, Gallego Barrero M, Murciano Gonzalo F, Sánchez Pasto E. N008. Reasons for consultation of patients with inflammatory bowel disease in telephone helplines attended by nurses. J Crohns Colitis. 2016;10 (suppl_1):S498. [DOI] [Cited in This Article: ] |
| 56. | Torrejón Herrera A, Masachs Peracaula M, Borruel Sainz N, Castells Carner I, Castillejo Badía N, Malagelada Benaprés JR, Casellas Jordá F. [Application of a model of continued attention in inflammatory bowel disease: the Crohn-colitis care unit]. Gastroenterol Hepatol. 2009;32:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Singh S, Brill JV, Proudfoot JA, Metcalfe L, Vu L, Sandborn WJ, Kosinski LR. Project Sonar: A Community Practice-based Intensive Medical Home for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2018;16:1847-1850.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Casey M, Hayes PS, Heaney D, Dowie L, Ólaighin G, Matero M, Hun S, Knarvik U, Alrutz K, Eadie L, Glynn LG. Implementing transnational telemedicine solutions: a connected health project in rural and remote areas of six Northern Periphery countries Series on European collaborative projects. Eur J Gen Pract. 2013;19:52-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Casellas-Jordá F, Borruel-Sainz N, Torrejón-Herrera A, Castells I. Effect upon hospital activity of the application of a continued care model centered on patients with inflammatory bowel disease. Rev Esp Enferm Dig. 2012;104:16-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Atreja A, Khan S, Rogers JD, Otobo E, Patel NP, Ullman T, Colombel JF, Moore S, Sands BE; HealthPROMISE Consortium Group. Impact of the Mobile HealthPROMISE Platform on the Quality of Care and Quality of Life in Patients With Inflammatory Bowel Disease: Study Protocol of a Pragmatic Randomized Controlled Trial. JMIR Res Protoc. 2015;4:e23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Coenen S, Nijns E, Weyts E, Geens P, Van den Bosch B, Vermeire S, Ferrante M, Vanhaecht K, Van Assche G. Development and feasibility of a telemonitoring tool with full integration in the electronic medical record: a proof of concept study for patients with inflammatory bowel disease in remission on biological therapy. Scand J Gastroenterol. 2020;55:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Del Hoyo J, Nos P, Bastida G, Faubel R, Muñoz D, Garrido-Marín A, Valero-Pérez E, Bejar-Serrano S, Aguas M. Telemonitoring of Crohn's Disease and Ulcerative Colitis (TECCU): Cost-Effectiveness Analysis. J Med Internet Res. 2019;21:e15505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Del Hoyo J, Aguas M. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clin Gastroenterol Hepatol. 2021;19:206-207. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 64. | de Jong MJ, Boonen A, van der Meulen-de Jong AE, Romberg-Camps MJ, van Bodegraven AA, Mahmmod N, Markus T, Dijkstra G, Winkens B, van Tubergen A, Masclee A, Jonkers DM, Pierik MJ. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18:1744-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Yao J, Fekadu G, Jiang X, You JHS. Telemonitoring for patients with inflammatory bowel disease amid the COVID-19 pandemic-A cost-effectiveness analysis. PLoS One. 2022;17:e0266464. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 66. | IMLCC. org. Interstate medical licensure compact. [Accessed 2022-12-09] Available from: http://www.imlcc.org/. [Cited in This Article: ] |
| 67. | CONGRESS. GOV. H.R.6074 - Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020 [Accessed 2022-12-09] Available from: https://www.congress.gov/bill/116th-congress/house-bill/6074. [Cited in This Article: ] |
| 68. | Avanesova AA, Shamliyan TA. Worldwide implementation of telemedicine programs in association with research performance and health policy. Health Policy Technol. 2019;8:179-191. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J Telemed Telecare. 2018;24:4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 773] [Cited by in F6Publishing: 811] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 70. | Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The Empirical Foundations of Telemedicine Interventions in Primary Care. Telemed J E Health. 2016;22:342-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 71. | Brooks E, Turvey C, Augusterfer EF. Provider barriers to telemental health: obstacles overcome, obstacles remaining. Telemed J E Health. 2013;19:433-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 72. | Broens TH, Huis in't Veld RM, Vollenbroek-Hutten MM, Hermens HJ, van Halteren AT, Nieuwenhuis LJ. Determinants of successful telemedicine implementations: a literature study. J Telemed Telecare. 2007;13:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 73. | Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2377] [Cited by in F6Publishing: 2345] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 74. | Martz E. Defining Self-Management on the Individual Level. In: Martz E (ed.). Promoting Self-Management of Chronic Health Conditions: Theories and Practice. New York, Oxford Academic, 2017: 10-28. [DOI] [Cited in This Article: ] |
| 75. | U. ; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 862] [Cited by in F6Publishing: 2380] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 76. | Bennebroek Evertsz' F, Nieuwkerk PT, Stokkers PC, Ponsioen CY, Bockting CL, Sanderman R, Sprangers MA. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7:890-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Bennebroek Evertsz' F, Hoeks CC, Nieuwkerk PT, Stokkers PC, Ponsioen CY, Bockting CL, Sanderman R, Sprangers MA. Development of the patient Harvey Bradshaw index and a comparison with a clinician-based Harvey Bradshaw index assessment of Crohn's disease activity. J Clin Gastroenterol. 2013;47:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Echarri A, Vera I, Ollero V, Arajol C, Riestra S, Robledo P, Calvo M, Gallego F, Ceballos D, Castro B, Aguas M, García-López S, Marín-Jiménez I, Chaparro M, Mesonero P, Guerra I, Guardiola J, Nos P, Muñiz J. The Harvey-Bradshaw Index Adapted to a Mobile Application Compared with In-Clinic Assessment: The MediCrohn Study. Telemed J E Health. 2020;26:80-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Marín-Jiménez I, Nos P, Domènech E, Riestra S, Gisbert JP, Calvet X, Cortés X, Iglesias E, Huguet JM, Taxonera C, Fernández R, Carpio D, Gutiérrez A, Guardiola J, Laria LC, Sicilia B, Bujanda L, Cea-Calvo L, Romero C, Rincón Ó, Juliá B, Panés J. Diagnostic Performance of the Simple Clinical Colitis Activity Index Self-Administered Online at Home by Patients With Ulcerative Colitis: CRONICA-UC Study. Am J Gastroenterol. 2016;111:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Larsen L, Drewes AM, Fallingborg J, Jacobsen BA, Jess T. Touch screens as a tool in patient care in the IBD outpatient clinic. Scand J Gastroenterol. 2016;51:1106-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Turner D, Griffiths AM, Mack D, Otley AR, Seow CH, Steinhart AH, Silverberg MS, Hyams J, Guyatt GH. Assessing disease activity in ulcerative colitis: patients or their physicians? Inflamm Bowel Dis. 2010;16:651-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 589] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 83. | Jairath V, Khanna R, Zou GY, Stitt L, Mosli M, Vandervoort MK, D'Haens G, Sandborn WJ, Feagan BG, Levesque BG. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 84. | Van Deen WK, van der Meulen-de Jong AE, Parekh NK, Kane E, Zand A, DiNicola CA, Hall L, Inserra EK, Choi JM, Ha CY, Esrailian E, van Oijen MG, Hommes DW. Development and Validation of an Inflammatory Bowel Diseases Monitoring Index for Use With Mobile Health Technologies. Clin Gastroenterol Hepatol. 2016;14:1742-1750.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Alcalá MJ, Casellas F, Fontanet G, Prieto L, Malagelada JR. Shortened questionnaire on quality of life for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:383-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Chen XL, Zhong LH, Wen Y, Liu TW, Li XY, Hou ZK, Hu Y, Mo CW, Liu FB. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual Life Outcomes. 2017;15:177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 87. | Yoon JY, Shin JE, Park SH, Park DI, Cha JM. Disability due to Inflammatory Bowel Disease Is Correlated with Drug Compliance, Disease Activity, and Quality of Life. Gut Liver. 2017;11:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | de Jong MJ, Roosen D, Degens JHRJ, van den Heuvel TRA, Romberg-Camps M, Hameeteman W, Bodelier AGL, Romanko I, Lukas M, Winkens B, Markus T, Masclee AAM, van Tubergen A, Jonkers DMAE, Pierik MJ. Development and Validation of a Patient-reported Score to Screen for Mucosal Inflammation in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Ehrmeyer SS, Laessig RH. Point-of-care testing, medical error, and patient safety: a 2007 assessment. Clin Chem Lab Med. 2007;45:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 92. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 93. | Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet V, Vavricka SR. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 94. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 95. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 96. | Aggarwal V, Day AS, Connor S, Leach ST, Brown G, Singh R, Friedman A, Zekry A, Craig PI. Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn's disease to assess remission and predict relapse. Gastrointest Endosc. 2017;86:1070-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, Flamant M, Savoye G, Jian R, Devos M, Porcher R, Paintaud G, Piver E, Colombel JF, Lemann M; Groupe D'etudes Thérapeutiques Des Affections Inflammatoires Digestives. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63-70.e5; quiz e31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 98. | Molander P, Färkkilä M, Ristimäki A, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M, Haapamäki J, Arkkila P, Nieminen U, Kuisma J, Punkkinen J, Kolho KL, Mustonen H, Sipponen T. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Solitano V, Alfarone L, D'Amico F, Peyrin-Biroulet L, Danese S. IBD goes home: from telemedicine to self-administered advanced therapies. Expert Opin Biol Ther. 2022;22:17-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 100. | Haisma SM, Galaurchi A, Almahwzi S, Adekanmi Balogun JA, Muller Kobold AC, van Rheenen PF. Head-to-head comparison of three stool calprotectin tests for home use. PLoS One. 2019;14:e0214751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 101. | Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, Marker D, Carlsen K, Burisch J, Munkholm P. Fecal Calprotectin Measured By Patients at Home Using Smartphones--A New Clinical Tool in Monitoring Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:336-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 102. | Kneepkens EL, Pouw MF, Wolbink GJ, Schaap T, Nurmohamed MT, de Vries A, Rispens T, Bloem K. Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol. 2017;83:2474-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Berends SE, D'Haens GRAM, Schaap T, de Vries A, Rispens T, Bloem K, Mathôt RAA. Dried blood samples can support monitoring of infliximab concentrations in patients with inflammatory bowel disease: A clinical validation. Br J Clin Pharmacol. 2019;85:1544-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Berends SE, Bloem K, de Vries A, Schaap T, Rispens T, Strik AS, Talwar R, Löwenberg M, D'Haens GR, Mathôt RA. Monitoring of Adalimumab Concentrations at Home in Patients with Inflammatory Bowel Disease Using Dried Blood Samples. Ther Drug Monit. 2020;42:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | DeVore AD, Wosik J, Hernandez AF. The Future of Wearables in Heart Failure Patients. JACC Heart Fail. 2019;7:922-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 106. | Hirten RP, Stanley S, Danieletto M, Borman Z, Grinspan A, Rao P, Sauk J, Chang L, Arnrich B, Bӧttinger E, Keefer L, Sands BE. Wearable Devices Are Well Accepted by Patients in the Study and Management of Inflammatory Bowel Disease: A Survey Study. Dig Dis Sci. 2021;66:1836-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Rowan C, Hirten R. The future of telemedicine and wearable technology in IBD. Curr Opin Gastroenterol. 2022;38:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Yvellez O, Andersen MJ Jr, Golan MA, Rodriquez R, Zmeter N, El Jurdi K, Rubin DT. P051 IBD patient compliance with mobile and wearable technologies as tools to assess quality of life, sleep quality and pain. Inflamm Bowel Dis. 2018;24:S18-S19. [DOI] [Cited in This Article: ] |
| 109. | Sossenheimer PH, Yvellez OV, Andersen M Jr, Pearl T, El Jurdi K, Rubin DB, Mayampurath A, Rubin DT. P579 wearable devices can predict disease activity in inflammatory bowel disease patients. J Crohns Colitis. 2019;13:S404. [DOI] [Cited in This Article: ] |
| 110. | Hirten RP, Danieletto M, Scheel R, Shervey M, Ji J, Hu L, Sauk J, Chang L, Arnrich B, Bӧttinger E, Dudley J, Keefer L, Sands BE. Longitudinal Autonomic Nervous System Measures Correlate With Stress and Ulcerative Colitis Disease Activity and Predict Flare. Inflamm Bowel Dis. 2021;27:1576-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Yi Y, Sossenheimer PH, Erondu AI, Skowron KB, Rai V, Singer JM, El Jurdi K, Hyman NH, Rubin DT. Using Wearable Biosensors to Predict Length of Stay for Patients with IBD After Bowel Surgery. Dig Dis Sci. 2022;67:844-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Walsh A. TrueColours: Real Time Data Collection in Patients With Ulcerative Colitis [doctoral thesis]. Oxford, UK: University of Oxford; 2017. [Accessed 2022-10-21] Available from: https://ora.ox.ac.uk/objects/uuid:a4ab55af-5364-4fa4-86ca-e84b917d7f70/download_file?file_format=pdf&safe_filename=DPhil%2BWALSH%2BTRUECOLOURS%2BFEB%2B2018.pdf&type_of_work=Thesis. [Cited in This Article: ] |
| 113. | Cross RK, Finkelstein J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: a 6-month pilot study. Dig Dis Sci. 2007;52:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Cross RK, Cheevers N, Finkelstein J. Home telemanagement for patients with ulcerative colitis (UC HAT). Dig Dis Sci. 2009;54:2463-2472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Elkjaer M, Burisch J, Avnstrøm S, Lynge E, Munkholm P. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Atreja A, Otobo E, Szigethy E, Shroff H, Chang H, Keefer L, Rogers J, Ullman T, Marion JF, Cohen B, Maser E, Itzkowitz S, Colombel JF, Sands B. DOP069 improved quality of care and quality of life for IBD patients using mobile based remote monitoring platform: A randomised control trial. J Crohns Colitis. 2018;12 (supplement_1):S077-S078. [DOI] [Cited in This Article: ] |
| 117. | Shah KP, Triana AJ, Gusdorf RE, McCoy AB, Pabla B, Scoville E, Dalal R, Beaulieu DB, Schwartz DA, Griffith ML, Horst SN. Demographic Factors Associated With Successful Telehealth Visits in Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. 2022;28:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |